Writing Chemical Formulae
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Chemical Formulae
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're focusing on the chemical formulae of different compounds. Who can tell me what a chemical formula represents?

It shows the elements in the compound and how many of each there are!

Yes! It’s like a recipe for combining ingredients in chemistry.

Great analogy! So, when we write a chemical formula, what rule must we keep in mind?

We need to balance the charges of the elements!

Exactly! Remember the acronym C.E.R. — Charges must Equalize for balance. Let's dive deeper into the general rules for writing these formulae.
Binary Compounds and Valencies
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, let's talk about binary compounds. What do we mean by binary?

Compounds that consist of two different elements!

Correct! So, when we write the formula for a binary compound, such as sodium chloride, what must we know?

The valences of sodium and chlorine!

Exactly! Sodium has a valency of +1 and chlorine has a valency of -1. So, what is the formula?

NaCl!

Perfect! And to sum up, we can use the acronym V.B.C., Valencies must Be Crossed, for binary compounds.
Using Polyatomic Ions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What happens when we encounter polyatomic ions in our compounds, like sulfate or hydroxide?

We have to consider their groups as a single unit with a specific charge!

Great! When writing the formula for calcium hydroxide, what do we need to include?

We need to write Ca(OH)₂ because we have two hydroxide ions.

Excellent! The brackets help signify that two hydroxide ions are present. Remember, P.A.R. — Polyatomic Must Always be Recognized!
Practice with Examples
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's practice a few examples. What is the formula for magnesium chloride?

It’s MgCl₂ since magnesium has a +2 valency and chlorine has a -1.

Exactly! Now, how about aluminum sulfate?

It’s Al₂(SO₄)₃ because there are three sulfate ions for every two aluminum ions!

Very well done! Remeber, rules in chemistry can help us predict and calculate. Let’s wrap up with a summary!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section details the process of writing chemical formulae for binary and ionic compounds, emphasizing the importance of balancing charges and following specific conventions for representing elements and their quantities in compounds.
Detailed
Writing Chemical Formulae
In this section, we explore the systematic approach to writing chemical formulae, which act as symbolic representations of the composition of compounds.
A chemical formula indicates the elements present in a compound and the ratio of their atoms. To ensure that the compound's overall charge is neutral, one must consider the valencies or charges of the elements involved. The section outlines specific rules for writing formulae, particularly for binary compounds, which consist of two different elements, as well as for compounds that contain polyatomic ions.
Key steps include:
- Identifying the Constituents: Recognize the metal and non-metal elements in the compound.
- Valency Usage: The valency, which indicates an element's combining capacity, plays a crucial role in determining the ratios used in the formula.
- Charge Balancing: The positive and negative charges of the elements must balance for the formula to be accurate. When combining elements, if the valency of one is not equal to the other, the numbers must be adjusted, often by criss-crossing the valences.
Incorporating brackets is essential when multiple ions of a polyatomic group are involved, clarifying the molecular structure of compound formulae. The section includes examples such as writing the formula for magnesium chloride and using comparative ratios for common substances.
The knowledge of chemical formulae not only serves as a foundation for further studies in chemistry but also illustrates the interactions and relationships between elemental substances.
Youtube Videos
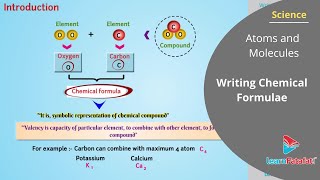

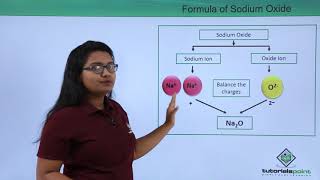



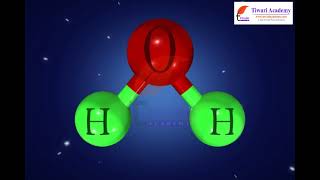


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Chemical Formulae
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The chemical formula of a compound is a symbolic representation of its composition.
Detailed Explanation
A chemical formula serves as a shorthand way to represent the elements in a compound and their proportions. For example, H₂O represents water, indicating it consists of two hydrogen atoms for every one oxygen atom.
Examples & Analogies
Think of a recipe for a cake. The recipe might say, '2 cups of flour, 1 cup of sugar, and 2 eggs.' Similarly, a chemical formula gives you the 'recipe' for a compound—how many of each type of atom is needed.
Valencies and Charges
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The valencies or charges on the ion must balance.
Detailed Explanation
In writing chemical formulas, the total positive charge from the metal or cation must equal the total negative charge from the non-metal or anion. This is necessary to create a neutral compound. For example, in sodium chloride (NaCl), sodium has a charge of +1 and chloride has a charge of -1, which balance each other out.
Examples & Analogies
Imagine a balance scale at a market. You cannot leave the market unless the scale is balanced, just like a chemical formula must balance its positive and negative charges before it is complete.
Order of Elements in Formulas
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When a compound consists of a metal and a non-metal, the name or symbol of the metal is written first.
Detailed Explanation
In chemical nomenclature, we always list metals before non-metals. This rule helps identify the type of compound and its properties. For example, in calcium oxide (CaO), calcium (metal) comes before oxygen (non-metal).
Examples & Analogies
Imagine a naming system for teams where the captain's name always comes first. In a chemical formula, the metal acts like the team captain and is always mentioned before the non-metal.
Using Brackets for Polyatomic Ions
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In compounds formed with polyatomic ions, the number of ions present in the compound is indicated by enclosing the formula of the ion in a bracket and writing the number of ions outside the bracket.
Detailed Explanation
Polyatomic ions are groups of atoms that carry a charge. When these ions are part of a compound and there is more than one of the ion type, we use brackets to indicate this. For instance, in magnesium hydroxide, the formula is Mg(OH)₂, denoting that there are two hydroxide ions for each magnesium ion.
Examples & Analogies
Think of a family with multiple children. If you wanted to specify that a certain number of children attend a camp, you might say, 'There are two children (the group) from the family.' The bracket groups the children together, just like it does for polyatomic ions.
Writing Formulas for Simple Compounds
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The simplest compounds, which are made up of two different elements, are called binary compounds.
Detailed Explanation
Binary compounds consist of two types of elements bonded together. To write their formula, you need to look at the elements involved and their valencies. For example, with hydrogen (H) which has a valency of +1 and chlorine (Cl) which has a valency of -1, the resulting compound hydrogen chloride (HCl) combines them in equal proportions.
Examples & Analogies
Just like mixing two colors of paint—if you mix red and blue evenly, you get purple—you can combine two elements in the right proportions to form a new compound.
Examples of Formulas
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Examples include:
(a) Formula for aluminium oxide: Al₂O₃
(b) Formula for calcium oxide: CaO
(c) Formula for sodium nitrate: NaNO₃
(d) Formula for calcium hydroxide: Ca(OH)₂
Detailed Explanation
Writing chemical formulas involves determining the correct ratios and charges of the combining elements. In Al₂O₃, there are two aluminum ions for every three oxide ions, maintaining charge balance in the compound. Similarly, Ca(OH)₂ indicates one calcium ion combines with two hydroxide ions.
Examples & Analogies
Picture adjusting a recipe with different measurements. If you use two cups of one ingredient and three of another, the measurements must add up properly to ensure your cake tastes right. Here, the charges must also align correctly to form a compound.
Key Concepts
-
Chemical Formula: Representation of the elements in a compound and their quantity.
-
Valency: The capacity of an atom to combine with other atoms.
-
Polyatomic Ions: Ions composed of two or more atoms bonded together.
-
Neutral Compound: A compound with balanced positive and negative charges.
-
Binary Compound: A compound made up of only two elements.
Examples & Applications
The formula for water (H₂O) indicates 2 Hydrogen atoms and 1 Oxygen atom.
Calcium Chloride (CaCl₂) shows 1 calcium ion and 2 chloride ions, balancing the charges.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Sodium and chloride, when they align, form NaCl, it's simply divine!
Stories
Imagine two friends, Sodium and Chlorine, who both want to create a balanced home. Sodium says he has one positive charge, while Chlorine brings one negative charge. Together, they form NaCl, a happy partnership!
Memory Tools
S.C.C. — Sodium and Chlorine Combine for Neutrality.
Acronyms
B.A.L.A.N.C.E. — Binary Atoms Link Arguably Non-charge Entities.
Flash Cards
Glossary
- Chemical Formula
A symbolic representation of the composition of a compound, indicating the elements and their ratios.
- Valency
The measure of an element's ability to combine with other elements, usually represented as a positive or negative charge.
- Polyatomic Ion
A charged group of two or more atoms bonded together which can act as a single ion.
- Binary Compound
A compound that consists of two different elements.
- Neutral Compound
A compound in which the total positive charges equal the total negative charges, resulting in no overall charge.
Reference links
Supplementary resources to enhance your learning experience.
