BOHR'S MODEL OF AN ATOM
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Bohr's Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore Niels Bohr's model of the atom, which revolutionized our understanding of atomic structure. Can anyone tell me why Rutherford's model needed improvement?

Rutherford's model couldn’t explain how electrons remain stable in their orbits.

Exactly! Bohr addressed this by proposing discrete electron orbits. What do you think 'discrete' means in this context?

I think it means that electrons can only be in specific orbits, not just anywhere.

Correct! Let's remember this with the acronym 'DES': Discrete Electron Shells. Bohr emphasized that only certain orbits are allowed.
Stability of Electrons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand that electrons occupy discrete orbits, why do you think this prevents them from losing energy?

Because they’re in stable paths, they don't need to lose energy like a moving car would if it’s running out of gas.

Great analogy! Let’s summarize with a mnemonic: 'STAY' - Stability Through Allowed Yields. This means electrons stay stable in allowed orbits.

So, electrons don't spiral into the nucleus, right?

Exactly! This stability explains the existence of matter as we know it.
Comparing Models
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How can we compare Bohr’s model to Rutherford’s?

Rutherford had a nucleus, but he didn’t explain why electrons don’t crash into it.

That's right! And how does Bohr add to this?

Bohr shows that electrons have fixed paths and don’t radiate energy.

Correct! This means that his model is more accurate for explaining stability in atoms.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
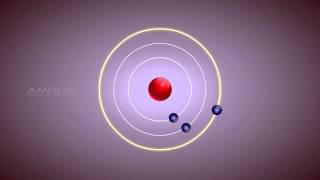
Niels Bohr proposed a revolutionary model of the atom that allowed for discrete electron orbits, explaining the stability of atoms by stating that electrons do not radiate energy while in these orbits. This model helped resolve the limitations of Rutherford’s atomic model and was foundational to modern atomic theory.
Detailed
Bohr's Model of an Atom
Niels Bohr made significant advancements in atomic theory by proposing a model that introduced quantized electron orbits. According to Bohr:
- Discrete Orbits: Electrons can only occupy certain stable orbits around the nucleus, called discrete energy levels. Unlike earlier beliefs, not every orbit is allowed.
- Energy Conservation: While orbiting in these defined paths, electrons do not emit energy, thereby retaining their stability contrary to what classical physics suggests.
- Significance: Bohr's model addressed major objections to Rutherford's model. It explained how atoms remain stable and formed the basis for further developments in quantum mechanics.
In summary, Bohr’s atomic model brought clarity to the arrangement of electrons and led to a deeper understanding of atomic behavior, rooted in quantized energy states.
Youtube Videos







Key Concepts
-
Discrete Electrons: Electrons inhabit specific orbits.
-
Energy Levels: Different levels correlate with the energy an electron possesses.
-
Atomic Stability: Stability arises from electrons maintaining their orbits without losing energy.
Examples & Applications
Example of Bohr's Model: An electron in a hydrogen atom occupies discrete energy levels, notably the K shell.
Comparison with Rutherford: Unlike Rutherford's model where electrons could spiral into the nucleus, Bohr explicitly states electrons remain in their orbits.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the shell where electrons rest, in quantized paths, they do their best.
Stories
Once upon a time, electrons lived in their own special orbits around the nucleus, never falling in, because they were told to stay and play in their defined paths.
Memory Tools
Remember 'DESS': Discrete Energy Shells Stable, to recall Bohr’s key points!
Acronyms
B.O.H.R.
Bohr's Orbits Have Rules.
Flash Cards
Glossary
- Discrete Orbits
Specific paths electrons can occupy around the nucleus, as proposed in Bohr's model.
- Atomic Stability
The concept that atoms do not undergo spontaneous change or reaction due to stable electron configurations.
- Energy Levels
Quantized levels of potential energy that electrons inhabit around the nucleus of an atom.
Reference links
Supplementary resources to enhance your learning experience.
