RUTHERFORD'S MODEL OF AN ATOM
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Rutherford's Experiment
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to delve into Rutherford's groundbreaking experiment involving gold foil and alpha particles. Can anyone tell me what alpha particles are?

Aren't they helium nuclei? They have a positive charge?

Exactly, great job! Rutherford directed alpha particles at a very thin layer of gold foil. Most of the particles passed through, but some were deflected. What do you think that indicates about the structure of the atom?

It suggests that there’s a lot of empty space in the atom!

Right! Only a small fraction of the alpha particles rebounded directly back to the source. This led Rutherford to propose that there is a tiny, dense center within the atom, called the nucleus. How do we think he decided the nucleus is positively charged?

Because the alpha particles were deflected, which are positively charged themselves, so they must be repelled by something positive in the nucleus!

Exactly, and that leads us to the idea that electrons orbit around this nucleus. Let's summarize: what did Rutherford conclude from his experiment?

Most of the atom is empty space, and the positive charge is concentrated in the nucleus!
Nitpicking Rutherford's Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've explored Rutherford's contributions, let’s talk about the limitations of his model. Can anyone think of what problems it might have?

Well, if electrons are orbiting the nucleus, shouldn't they lose energy and spiral into it?

Exactly! That’s one of the key issues. Electrons in orbit should radiate energy due to acceleration, ultimately leading to instability. This contradiction prompted further inquiry. Who can tell me which scientist built upon Rutherford’s findings?

Niels Bohr, right? He proposed that electrons occupy specific energy levels!

Spot on! Bohr's model clarified the arrangement of electrons and their energy levels within an atom. So, what were the takeaways from Rutherford’s model despite its limitations?

He showed that there’s a nucleus at the center of the atom, which was new information!

And it helped lead to the development of more accurate atomic models later.
The Nucleus and its Components
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dig deeper into what makes up this nucleus. Can anyone tell me what particles reside in the nucleus?

Protons and neutrons!

Correct! Protons have a positive charge, while neutrons are neutral. Can anyone tell me why neutrons are important?

They help stabilize the nucleus by offsetting the repulsion between protons!

Absolutely right! The balance of protons and neutrons is what keeps the nucleus stable. Now, the mass of the atom largely comes from the nucleus. Can anyone share how this influences the overall structure of the atom?

Since most of the mass is in the nucleus, the electrons must be very light compared to them!

Exactly! Electrons have about 1/2000th the mass of a proton. Let’s summarize this session: what did we learn about the components of the nucleus?

The nucleus contains protons and neutrons, with neutrons helping to stabilize protons and contributing to the atom's mass!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
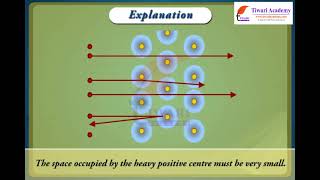
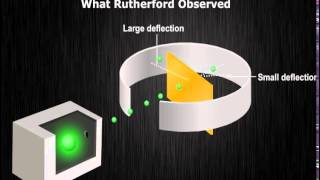
Ernest Rutherford's experiments led to the identification of the atomic nucleus, shifting the view of atomic structure. His alpha-particle scattering experiment revealed that the atom consists mostly of empty space, with a small, dense, positively charged nucleus around which electrons orbit.
Detailed
Ernest Rutherford's research fundamentally changed the prevailing model of atomic structure. Through his gold foil experiment, he observed the behavior of alpha particles and concluded that most of an atom is empty space, with a dense nucleus comprised of protons at its core. He proposed that electrons orbit this nucleus much like planets orbit the sun. The model suggested that the mass of the atom resides primarily in the nucleus, while electrons exist in specific energy levels around it. However, Rutherford's model could not explain why atoms are stable, as electrons should radiate energy and spiral into the nucleus. Hence, while his discovery was monumental, it laid the groundwork for further developments in atomic theory, particularly that by Niels Bohr.
Youtube Videos





Key Concepts
-
Atomic Nucleus: A small, dense region containing protons and neutrons, from which most of the atom's mass is derived.
-
Rutherford's Experiment: Demonstrated that atoms consist mostly of empty space, altering the view of atomic structure.
-
Subatomic Particles: Protons (positive charge), neutrons (neutral), and electrons (negative charge) are the building blocks of atoms.
Examples & Applications
In Rutherford's gold foil experiment, most alpha particles passed through the gold foil, indicating that an atom is mostly empty space.
The dense nucleus at the center containing protons and neutrons accounts for most of the atomic mass.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Rutherford took his aim, at gold foil, to frame, with alpha rays in flight, revealed the atom's might.
Stories
Once upon a time, in the atomic realm, Rutherford explored. Armed with alpha particles, he sailed through gold wafers, only to find a hidden, dense nucleus where protons and neutrons would rest, surrounded by the orbiting electrons, keeping the structure of matter.
Memory Tools
PEN for remembering: P = Protons, E = Electrons, N = Neutrons.
Acronyms
N.P.E for remembering nucleons
for Neutrons
for Protons
for Electrons.
Flash Cards
Glossary
- Alpha Particle
A positively charged particle made up of two protons and two neutrons, emitted during certain types of radioactive decay.
- Nucleus
The dense, positively charged center of an atom that contains protons and neutrons.
- Electron
A negatively charged subatomic particle that orbits the nucleus of an atom.
- Proton
A positively charged subatomic particle found in the nucleus of an atom.
- Neutron
A neutral subatomic particle found in the nucleus of an atom.
Reference links
Supplementary resources to enhance your learning experience.
