Valency
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Valency
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Hello class! Today, we're going to talk about valency, which is the combining capacity of an atom. Can anyone tell me what they think valency might relate to?

Is it how well an atom can combine with other atoms?

Exactly, Student_1! Valency reflects how many electrons an atom can lose, gain, or share to achieve a full outermost shell. Now, what do you think determines how many electrons are in that outer shell?

Is it the atomic number?

Good point, Student_2! The atomic number defines the number of protons, and in a neutral atom, protons equal electrons. So, the arrangement of electrons tells us a lot about valency. Let's remember that 8 is great for stability due to the octet rule!

But what about elements that have fewer than 8 electrons?

Great question, Student_3! Atoms with fewer than 8 electrons will tend to react to gain stability. This gives them a defined valency.

In summary, valency indicates how an atom will interact based on its electron arrangement. To remember this, think of the saying, 'Valency holds the key to bond and free!'
Explaining Valency with Examples
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss some specific examples of valency. For instance, take oxygen. Can anyone tell me how many electrons are in its outer shell?

Oxygen has 6 electrons in its outer shell!

Correct, Student_4! Oxygen needs 2 more electrons to fulfill the octet rule. Thus, its valency is 2 because it looks to gain two electrons. Can anyone tell me about sodium?

Sodium has 1 electron in its outer shell, so its valency is 1!

Right again! Sodium will lose that electron to achieve stability. Let’s remember that sodium is very reactive due to this valency. Quick question for you all: If an atom has 7 valence electrons, what would its valency be?

Its valency would be 1, since it needs 1 more to reach 8!

Well done, class! Remember, the way we determine valency is closely tied to the arrangement of electrons in the outer shell, which is crucial for understanding chemical reactions. For our next summary, let's say, 'Valency is key, reactively free!'
Valency and Chemical Reactivity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How does valency influence how atoms react with each other? Let’s explore this together. Why do you think elements with a full outer shell are less reactive?

Because they don't need to gain or lose any electrons?

Good insight, Student_3! Elements like noble gases have full outer shells, which makes them stable and not likely to react. But what about other elements?

They will either lose, gain, or share electrons to become stable, depending on their valency!

That’s absolutely right! Understanding valency helps us predict how elements will combine. As an aid, remember 'Valency decides, bonds on both sides.'

Let’s conclude this session with the key point: Elements with similar valencies often react in predictable patterns. For example, metals typically lose electrons, whereas non-metals tend to gain them during reactions.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section introduces the concept of valency, describing how the number of valence electrons determines an atom's ability to bond with other atoms. The section explains the significance of electron arrangement in determining chemical reactivity and stability.
Detailed
Understanding Valency
Valency is the measure of an atom's ability to engage in chemical bonding with other atoms and is determined by the number of electrons in its outermost shell—known as valence electrons. The outermost shell can hold a maximum of 8 electrons, following the octet rule. Atoms strive for a full outer shell, leading to stability and lower reactivity. Elements with a full outer shell exhibit minimal chemical activity, while those with incomplete shells actively lose, gain, or share electrons to achieve stability. For example:
- Valency of Hydrogen (H): 1
- Valency of Magnesium (Mg): 2
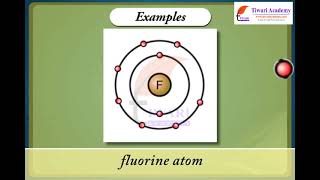
- Valency of Chlorine (Cl): 1
Through this section, we understand how the valency of elements is linked to their electron configuration, with practical applications in predicting how different elements will combine chemically. The information provided is fundamental in both chemistry and understanding the nature of matter.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Valency
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We have learnt how the electrons in an atom are arranged in different shells/orbits. The electrons present in the outermost shell of an atom are known as the valence electrons.
Detailed Explanation
In an atom, electrons are organized into different layers or shells based on their energy levels. The outermost shell, which is the furthest from the nucleus, contains electrons that are crucial for the atom's chemical behavior. These outer electrons are called valence electrons because they determine how an atom can interact or bond with other atoms.
Examples & Analogies
Think of the outermost shell like the outermost layer of a layered cake. Just as the frosting on the top layer of cake determines how it tastes, the valence electrons determine how the atom will bond with others.
Stability of Valence Electrons
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
From the Bohr-Bury scheme, we also know that the outermost shell of an atom can accommodate a maximum of 8 electrons. It was observed that the atoms of elements, completely filled with 8 electrons in the outermost shell show little chemical activity.
Detailed Explanation
According to the Bohr-Bury model, atoms strive to fill their outermost electron shell, which can hold up to 8 electrons. Atoms with a full outer shell are chemically stable and tend not to react with other atoms. This is why noble gases, which have full outer shells, are largely unreactive and rarely form bonds with other elements.
Examples & Analogies
Imagine a classroom. If all the seats (electrons) in a room (outer shell) are filled, the students (atoms) are content and won’t be eager to interact with others. On the other hand, if there are empty seats, the students will seek out friends (other atoms) to sit together, resembling chemical bonding.
Valency of Elements
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Of these inert elements, the helium atom has two electrons in its outermost shell and all other elements have atoms with eight electrons in the outermost shell. The combining capacity of the atoms of elements, that is, their tendency to react and form molecules with atoms of the same or different elements, was thus explained as an attempt to attain a fully-filled outermost shell.
Detailed Explanation
Inert elements like helium have full outer shells, so they do not react with others. Elements that do not have a full outer shell will tend to gain, lose, or share electrons to reach stability. The number of electrons gained or lost helps define the element's valency—its ability to combine with other elements.
Examples & Analogies
Consider how people form teams based on shared interests. If some members are missing in a team (incomplete outer shell), they will invite others to join (gain or lose electrons) until the team is fully formed and functioning well (stable outer shell).
Calculating Valency
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An outermost-shell, which had eight electrons was said to possess an octet. Atoms would thus react, so as to achieve an octet in the outermost shell. This was done by sharing, gaining or losing electrons.
Detailed Explanation
The octet rule states that atoms are most stable when they have eight electrons in their outer shell (octet). If an atom starts with fewer than eight, it may either lose electrons, gain electrons, or share electrons with other atoms to achieve stability. The number of electrons lost or gained helps determine the valency of the element.
Examples & Analogies
Think of a bank. If you have only a little money (few electrons), you may either save money (gain electrons), spend some (lose electrons), or partner with a friend to pool resources (share electrons) to reach your financial goal of a comfortable savings balance (stable octet).
Examples of Valency
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For example, hydrogen/lithium/sodium atoms contain one electron each in their outermost shell, therefore each of them can lose one electron. So, they are said to have valency of one...
Detailed Explanation
Element valencies can be simple to determine. For elements like hydrogen, lithium, and sodium, which each have one electron in their outermost shell, they can easily lose that electron to achieve a stable configuration. Thus, they all share a valency of one since they can each bond with one other atom to reach stability.
Examples & Analogies
If you think about social interactions, people who are willing to bring one friend to a gathering (losing an electron) can easily form a bond with others. If someone is looking for friendship and brings one other person, their ability to form a connection can be thought of as their 'valency' in social terms.
Key Concepts
-
Valency: The capacity of an atom to bond based on electrons in its outer shell.
-
Outer Shell Electrons: Electrons in the outermost shell that determine reactivity.
-
Chemical Reactivity: How elements interact based on their valency.
Examples & Applications
The valency of sodium (Na) is 1 as it has one electron in its outer shell.
Oxygen (O) has a valency of 2 because it requires two electrons to complete its outer shell.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Valence, valence, find your balance, study the shell—avoid mischance!
Stories
Once a sodium atom was lonely with its single electron. It searched for a friend, found chlorine, and they formed a bond by sharing—what a blend!
Memory Tools
S.O.C. helps remember: Sodium loses One (1), Calcium loses Two (2), and Chlorine needs One (1) to achieve balance.
Acronyms
VALENCE - 'Valley of Atoms Linking Every Noble Element.'
Flash Cards
Glossary
- Valency
The combining capacity of an atom, based on the number of electrons in its outermost shell.
- Octet Rule
The principle stating that atoms tend to prefer having eight electrons in their valence shell.
- Valence Electrons
Electrons located in the outermost shell of an atom that are involved in chemical bonding.
Reference links
Supplementary resources to enhance your learning experience.
