Polymers
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Classification of Polymers
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss polymers and how they are classified. Can anyone tell me what a polymer is?

I think a polymer is a type of large molecule made from repeating units.

Exactly! Polymers are made up of monomers. Now, let's classify these polymers into three main types: thermoplastics, thermosets, and elastomers. Remember the acronym TTE — T for thermoplastics, T for thermosets, and E for elastomers. Can anyone give examples?

For thermoplastics, there’s polyethylene and PVC.

And epoxy and Bakelite are thermosets!

Natural rubber counts as an elastomer, right?

Correct! So remember TTE for easy recall of these polymer types. Let's move on to their applications in various industries.
Applications of Polymers
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know about classifications, let's talk about where polymers are used. Can anyone name some applications?

I think they are used in packaging?

Yes, that's right! Polymers are heavily used in packaging due to their lightweight and flexible nature. What else?

Electronics, like in circuit boards?

And how about in cars or automotive parts?

Absolutely! Polymers are found in textiles, automotive materials, and even medical devices due to their versatility. Imagine a world without polymers!
Polymerization Techniques
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's dive into how we actually make these polymers. Who can tell me the different polymerization techniques we've learned?

There are addition polymerization and condensation polymerization!

Perfect! Addition polymerization leads to polymers without byproducts. For example, can you name one?

Polyethylene!

Great! Now what about condensation polymerization?

Nylon is formed this way, right?

Exactly! This method releases byproducts like water. Lastly, what is copolymerization?

That's when two different monomers combine to create a new polymer.

Yes! Excellent work. Remember these techniques as they help us understand how to tailor polymer properties for specific needs.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Polymers are materials categorized into thermoplastics, thermosets, and elastomers. They are used across various industries, with distinct polymerization techniques leading to different types of polymers. Understanding these aspects is crucial for applications in packaging, electronics, and medical devices.
Detailed
Polymers
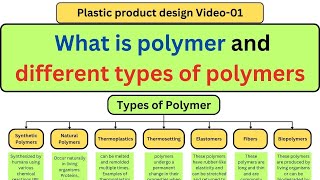
Polymers are large molecules formed by the repetition of smaller units known as monomers. They are classified into three primary categories based on their thermal properties:
- Thermoplastics: These polymers soften upon heating and can be remolded several times, making them versatile for various applications. Common examples include polyethylene and PVC.
- Thermosets: These types of polymers undergo a chemical change and harden irreversibly upon heating, which makes them suitable for products requiring durability, such as epoxy and Bakelite.
- Elastomers: This category includes polymers that exhibit significant elasticity. Natural rubber and neoprene are typical examples, often used in applications that require flexibility.
Applications
Polymers are prevalent in numerous fields such as packaging, electronics, automotive manufacturing, textiles, and medical devices due to their lightweight, versatile nature.
Polymerization Techniques
The process of forming polymers can vary:
- Addition Polymerization: Monomers join together without producing byproducts; polyethylene is a prime example.
- Condensation Polymerization: In this process, byproducts (like water) are formed; nylon is one of the well-known polymers created through this method.
- Copolymerization: This technique involves two or more different types of monomers combining to form a polymer, enhancing properties for specific applications.
Understanding the classification, applications, and polymerization methods of polymers provides a foundational comprehension of material science, essential for innovations and advancements in various technologies.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Classification of Polymers
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
a. Classification of Polymers
- Thermoplastics: Soften on heating, can be remolded (e.g., polyethylene, PVC)
- Thermosets: Irreversibly hardened upon heating (e.g., epoxy, bakelite)
- Elastomers: Highly elastic (e.g., natural rubber, neoprene)
Detailed Explanation
Polymers can be classified into three main types: thermoplastics, thermosets, and elastomers. Thermoplastics are materials that become soft when heated, allowing them to be reshaped multiple times. Common examples include polyethylene and PVC, which are often used in packaging and construction. In contrast, thermosets are polymers that undergo a chemical change when heated, making them rigid and unable to be remolded. Examples include epoxy and bakelite, which are utilized for durable products like electrical insulators. Lastly, elastomers are very flexible and have the ability to stretch significantly, returning to their original shape, much like rubber bands. Natural rubber and neoprene are types of elastomers used in various applications, from tires to wetsuits.
Examples & Analogies
Think of thermoplastics as playdough; when you heat it, you can reshape it into different forms. Thermosets are like a cake; once baked, you cannot change it back to batter. Elastomers are similar to a rubber band; when you stretch it, it returns to its original shape.
Applications of Polymers
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
b. Applications
- Used in packaging, electronics, automotive, textiles, and medical devices
Detailed Explanation
Polymers are extremely versatile materials used in a wide range of industries. In packaging, they provide lightweight, durable, and moisture-resistant options that help preserve food and products. In the electronics field, polymers are used for insulation and in components like circuit boards due to their electrical properties. The automotive industry relies on polymers to create lightweight parts that contribute to fuel efficiency and improved safety. Additionally, textiles use synthetic polymers to create a variety of fabrics for clothing, while in the medical sector, polymers are crucial for making devices like syringes, implants, and prosthetics due to their biocompatibility and sterilization capabilities.
Examples & Analogies
Imagine you go shopping. The plastic bag you use is made of polymers, protecting your items and keeping them dry. In your car, lightweight plastic materials help save fuel by making it lighter. In a hospital, the syringes used for vaccinations are also made of polymers, ensuring they are safe and easy to use.
Polymerization Techniques
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
c. Polymerization Techniques
- Addition Polymerization: Monomers add without byproducts (e.g., polyethylene)
- Condensation Polymerization: Byproducts (like water) are released (e.g., nylon)
- Copolymers: Two or more different monomers polymerized together
Detailed Explanation
There are several methods to create polymers, known as polymerization techniques. Addition polymerization involves combining monomers (the small molecules that make up polymers) without producing any byproducts. An example is polyethylene, used in plastic bags. Condensation polymerization, on the other hand, results in the formation of byproducts like water. A common example is nylon, which forms when two different monomers react. Lastly, copolymerization involves the combination of two or more distinct types of monomers, resulting in a copolymer that can possess varied properties suitable for specific applications.
Examples & Analogies
Think of addition polymerization like building a LEGO tower by stacking individual blocks (monomers) without losing any pieces. In contrast, condensation polymerization is like making a smoothie; while you're blending together fruits (monomers), you might also release some juice (byproduct) in the process. Copolymers can be compared to a recipe where you blend different ingredients—like chocolate and vanilla ice cream—together to create something uniquely delicious.
Key Concepts
-
Polymers: Large molecules formed from repeating units known as monomers.
-
Types of Polymers: Thermoplastics, thermosets, and elastomers, each with unique properties.
-
Applications: Polymers are extensively used in various industries.
-
Polymerization Techniques: Addition, condensation, and copolymerization are key methods in polymer formation.
Examples & Applications
Polyethylene used in plastic bags is a thermoplastic.
Bakelite, used in electrical insulators, is a thermoset.
Natural rubber, used in tires, represents an elastomer.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Polymers are great, both hard and soft, / Thermoplastics bend, while thermosets scoff.
Stories
Once in a materials lab, different polymers gathered: the flexible thermoplastics, the tough thermosets, and the rubbery elastomers, each telling tales of their uses.
Memory Tools
Remember TTE for Polymers: T for Thermoplastics, T for Thermosets, E for Elastomers.
Acronyms
To remember types, think TTE; Thermoplastics, Thermosets, and Elastomers, that's the key!
Flash Cards
Glossary
- Polymer
A large molecule composed of many repeated subunits called monomers.
- Thermoplastics
Polymers that soften upon heating and can be remolded repeatedly.
- Thermosets
Polymers that harden irreversibly when heated.
- Elastomers
Polymers with excellent elasticity, such as rubber.
- Polymerization
The process by which monomers are chemically bonded to form polymers.
- Addition Polymerization
A method where monomers add together without byproducts.
- Condensation Polymerization
A polymer formation method that releases byproducts, like water.
- Copolymers
Polymers made from two or more different types of monomers.
Reference links
Supplementary resources to enhance your learning experience.
