Water Quality Parameters
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Water Quality Parameters
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore Water Quality Parameters and their critical role in environmental health. Why do you think monitoring water quality is essential?

Because poor water quality can harm our health?

Exactly! Monitoring helps identify hazardous materials that could impact public health. Can anyone name a common exposure pathway?

Inhalation, I think?

Correct! Inhalation, ingestion, and skin contact are the three main pathways. To remember them, we can use the acronym **'IIS': Inhalation, Ingestion, Skin contact.**

What happens if hazardous substances enter our body?

Great question! They can trigger various health effects depending on their toxicity. Let's move on to how we measure these pollutants.
Exposure Pathways Explained
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's elaborate on the three exposure pathways. Can someone explain inhalation?

Inhalation occurs when we breathe in harmful substances in the air.

Correct! Ingestion takes place when we consume contaminated water or food. Lastly, dermal exposure happens through skin contact, which can be equally harmful. To remember, think of **'IIDS'** - Inhalation, Ingestion, Dermal contact!

Are there specific pollutants we should be concerned about when it comes to water quality?

Yes! Common pollutants include heavy metals, pathogens, and pesticides. Monitoring these substances helps us ensure safety.

How do we find out where these pollutants are coming from?

Excellent point! This leads us to investigate the sources of pollution. Let’s summarize: remember 'IIDS' for exposure pathways!
Sources and Transport of Pollutants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's consider how hazardous materials reach the environment. What might be a source of pollutants?

Manufacturing plants might release chemicals into the water.

Exactly! Sources can vary, including industrial processes like combustion. Once these chemicals are in the environment, they can move through air, water, and soil.

How do we track their movement?

We use fate and transport models to assess how pollutants behave after their release. Remember the concept of **'Fate and Transport'** in relation to pollutants!

So, understanding these factors can help us predict their impact?

Absolutely! It’s vital for risk assessment and making informed decisions about health.
Environmental Compartment Interactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's now look at how different environmental compartments interact, such as the transfer between water and soil. Why is that important?

Chemicals can spread between them, affecting overall quality!

Exactly! Contaminants in water can settle into sediments, which can later leach back into the water body. Think of this cycle as 'Continuous Interaction.'

That sounds complex, how do pollutants behave differently in each environment?

Great question! Some chemicals may evaporate in air or bind tightly to soil, affecting their transport. Utilizing **'Interphase Transport'** will help us track this!

So every compartment plays a role in pollution dynamics!

Exactly! Always remember that all compartments are interconnected.
Health Implications and Risk Assessment
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's discuss the health risks associated with exposure to contaminated water. Why do you think it's important to assess risk?

To protect public health!

Precisely! We must evaluate the **dosage and exposure levels** to understand potential health effects. Can anyone share a common concept related to this?

LD50?

That's right! LD50 defines the lethal dose for 50% of a population, and studying dose-response relationships helps anticipate health impacts. Let’s remember, **'Assess for Health Safeguard'**!

What actions can be taken to reduce these risks?

We can improve regulations, enhance monitoring, and raise public awareness. Remember that informed communities can advocate for safer environments!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Water Quality Parameters are critical in monitoring environmental health. This section explains various exposure pathways through which individuals may come into contact with hazardous materials found in water. The origin and transport mechanisms of these pollutants in different environmental compartments, as well as their health implications, are also explored.
Detailed
Water Quality Parameters
Understanding Water Quality Parameters is essential for assessing environmental health, as they directly affect human well-being. This section delves into the concept of water quality, identifying various exposure pathways such as inhalation, ingestion, and skin contact—each representing a method through which people can come into contact with hazardous substances.
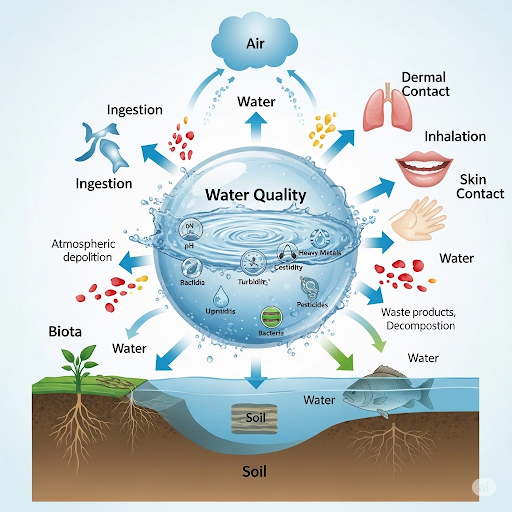
The chapter highlights exposure pathways linking hazardous materials to environmental compartments—air, water, soil, and biota. These pathways serve as vital indicators for identifying sources of pollution and understanding how these harmful substances affect human health through different environmental processes. Assessment methods for monitoring pollutants in water, soil, and air will be discussed, focusing on the relevant properties and behaviors of these pollutants as they move through and interact with various environmental phases. Ultimately, a comprehensive understanding of these parameters aids in risk assessment and the management of environmental impacts on health.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Water Quality
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Water quality is essential for both environmental and human health. It refers to the chemical, physical, biological, and radiological characteristics of water, usually in relation to its suitability for a particular purpose.
Detailed Explanation
Water quality is a measure of how clean or polluted water is. It affects all forms of life—plant and animal alike. Good water quality means that water is safe for drinking, swimming, and supporting aquatic ecosystems. Understanding these parameters helps us assess if the water is suitable for its intended use, such as drinking, agricultural irrigation, or supporting wildlife.
Examples & Analogies
Think of water quality like the cleanliness of a kitchen. If the kitchen is spotless, you can freely prepare food without worries. But if it's dirty, there could be germs that might make you sick. Similarly, clean water helps us stay healthy, while contaminated water can pose health risks.
Key Water Quality Parameters
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Several key parameters are used to assess water quality, including pH, turbidity, dissolved oxygen, and the presence of contaminants like heavy metals and pathogens.
Detailed Explanation
Key water quality parameters provide vital indicators of water conditions. For instance, pH measures how acidic or basic the water is, affecting everything from aquatic life to drinking water safety. Turbidity indicates how clear the water is, influencing light penetration necessary for aquatic plants. Dissolved oxygen is crucial for fish and other aquatic organisms. Heavy metals and pathogens refer to harmful substances that can contaminate water.
Examples & Analogies
Consider a garden. The soil's pH level affects how well plants grow, similar to how water's pH impacts aquatic plants and animals. Turbidity can be likened to the cloudiness of juice—it affects how much light reaches the plants in the water, just like too much dust could limit sunlight for the plants in your garden.
pH Levels in Water
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The pH of water is a measure of its acidity or alkalinity, ranging from 0 to 14, with 7 being neutral. Most aquatic life thrives at a pH between 6.5 and 8.5.
Detailed Explanation
Water's pH is critical because different aquatic species have different tolerances. If water becomes too acidic or too alkaline, it can harm organisms that depend on stable conditions for survival. For example, fish and frogs thrive in a balanced pH range, and deviations can lead to stress or mortality in these populations.
Examples & Analogies
Imagine baking a cake. If you don't get the balance right between acidic and alkaline ingredients, your cake might not rise or taste good. Similarly, maintaining the right pH balance in water helps keep aquatic ecosystems healthy.
Turbidity and its Effects
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Turbidity is the cloudiness of water caused by suspended particles. High turbidity can harm aquatic life by blocking sunlight and reducing photosynthesis.
Detailed Explanation
Turbidity affects water quality by limiting light availability for aquatic plants, which are essential to the food chain. High turbidity can also indicate pollution, as it is often associated with sediments from soil erosion, algae blooms, or discharges from wastewater. Less light affects plant growth, and in turn, less food is available for herbivorous aquatic animals.
Examples & Analogies
Consider murky water in a swimming pool—it can make it hard to see the bottom, indicating that the water is not clean. The same goes for lakes and rivers; if they are murky and full of particles, it limits the light necessary for aquatic ecosystems to flourish.
Dissolved Oxygen Levels
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Dissolved oxygen is vital for the survival of aquatic organisms. Low levels can lead to hypoxia, where aquatic life cannot survive.
Detailed Explanation
Dissolved oxygen is crucial for aquatic creatures like fish and invertebrates as they rely on it for respiration. Water can become poorly oxygenated due to several factors such as high temperatures, excessive algae growth, or organic matter decomposition, leading to conditions uninhabitable for most aquatic life, known as hypoxia. Monitoring this parameter helps in managing aquatic ecosystems effectively.
Examples & Analogies
Think of dissolved oxygen like the air we breathe. If you're in a crowded room with poor ventilation, it becomes hard to breathe. Similarly, fish and other aquatic organisms need sufficient oxygen to survive; too little oxygen leads to distress and death, similar to our own struggle in a stuffy room.
Contaminants of Concern
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Contaminants such as heavy metals, nutrients, and pathogens can severely impact water quality and pose health risks to humans and wildlife.
Detailed Explanation
Heavy metals like lead, mercury, and cadmium can accumulate in ecosystems, leading to toxicity in aquatic life and humans who consume them. Nutrient overloads, often from agricultural runoff, can lead to eutrophication, resulting in harmful algal blooms. Pathogens introduce risks of waterborne diseases, especially in drinking water sources, emphasizing the need for effective monitoring and management of these contaminants.
Examples & Analogies
Imagine a food chain that's been tainted by a toxic ingredient. If a farmer sprays harmful chemicals on their crops, and the runoff enters a nearby stream, the fish that live there can accumulate these toxins. When you eat that fish, you may also consume those harmful chemicals, similar to how bad food can affect your health.
Key Concepts
-
Water Quality Parameters: Critical for monitoring health impacts from pollutants in water.
-
Exposure Pathways: Routes to human exposure to hazardous substances, which include inhalation, ingestion, and skin contact.
-
Fate and Transport: The behavior of pollutants as they move through and interact with different environmental compartments.
Examples & Applications
Heavy metals like lead can enter drinking water supply through industrial discharges, affecting human health upon ingestion.
Pathogens can be transferred in water directly to humans, causing diseases when consumed or when the skin is exposed.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In water and soil, pollutants roam, in pathways they travel, wherever they call home.
Stories
Imagine a tiny molecule polluting a river, it flows into the soil and meets a thirsty plant, that plant shares it with a hungry animal, and together they reach a human being, leading to health concerns.
Memory Tools
Use 'IIS' to recall: Inhalation, Ingestion, Skin contact are the pathways for pollutants.
Acronyms
**'FAT'** for Fate and Transport
Followed
Adapted
Transformed as pollutants move through environments.
Flash Cards
Glossary
- Exposure Pathways
The routes through which hazardous substances enter the human body, primarily through inhalation, ingestion, and dermal contact.
- Hazardous Materials
Substances that can cause significant harm to human health or the environment due to their toxicity.
- Fate and Transport
The processes that determine the movement and transformation of pollutants from their source through the environment to exposure points.
- Ld50
The lethal dose required to kill 50% of a test population, often used in toxicology studies.
- Interphase Transport
The movement of pollutants between different environmental phases, such as air, water, and soil.
Reference links
Supplementary resources to enhance your learning experience.
