PM – Particulate Matter
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Particulate Matter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing Particulate Matter, or PM. PM is critical in evaluating air quality. Can anyone explain what particulate matter includes?

Is it just dust and smoke?

Great start! Yes, it includes soot, dust, and even liquid droplets from various sources, primarily combustion. Remember: PM stands for 'Particulate Matter.'

What are the different types?

Excellent question! We categorize PM mainly into PM10 and PM2.5. Can anyone tell me their sizes?

PM10 is less than 10 microns and PM2.5 is less than 2.5 microns.

Exactly! PM10 represents larger particles that can be trapped in the throat, whereas PM2.5 can reach the lungs.

So, PM2.5 is worse for health?

Correct! PM2.5 can evade the body's natural defenses and poses serious health risks. To remember this, think: 'PM2.5 – deep in the lungs, troubles come along!'

To summarize, particulate matter includes various particles, where size directly influences health effects.
Sources and Standards of Particulate Matter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, let's discuss how PM is generated. What are some common sources?

Cars, factories, and forest fires?

Correct! These combustion processes are significant sources. Remember that PM originates mainly from gas-phase reactions. Now, who can tell me about the standards set for PM?

I think the US EPA has guidelines on PM levels.

Right! The US EPA has detailed protocols for monitoring PM. It's essential to stay updated with these standards. For example, regulatory agencies worldwide adopt similar criteria for PM assessment.

What do they mean by aerodynamic diameter?

Aerodynamic diameter refers to a dimension of particles that equate their settling velocities. It's crucial because different shapes and densities affect how particles behave in the air. An easy way to think of it is: 'aerodynamics - how it flies!'

In summary, understanding the sources of PM and regulatory standards guides us in controlling air quality.
Health Implications of Particulate Matter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s explore why particle sizes matter. What happens in our bodies when inhaling different PM sizes?

Maybe smaller ones can go deeper into the lungs?

Absolutely! PM2.5 can penetrate deep into lung tissue, while PM10 tends to get trapped in the upper respiratory tract. Can anyone share why this is concerning?

Because PM2.5 can cause serious health issues?

Exactly! They can lead to conditions like asthma and cardiovascular problems. Remember: 'Small particles, big problems!'

And the way they settle in our respiratory system is influenced by their mass and shape, right?

Correct! Inertia plays a significant role in whether they get deposited or not. To summarize, smaller particles pose greater health risks due to their ability to reach critical areas in our lungs.
Future Directions in PM Research and Regulation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As our understanding of PM evolves, what do you think will change in health regulations?

Maybe the standards will be stricter for smaller particles?

Exactly! New research will lead to updated guidelines. With advancements in measurement technology, we will better understand nanoparticle impacts.

Are there already new technologies for measuring particles?

Indeed! Emerging methods allow us to detect nanoparticles more effectively. Remembering this is essential: 'New tech, new insights!'

So, we need ongoing research to keep up with these developments?

Absolutely! Until comprehensive methods to measure these nanoparticles are accessible, regulations will continue to evolve slowly. In summary, future research will shape our understanding and the regulatory landscape of PM.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Particulate matter (PM) plays a crucial role in evaluating air quality. The section defines PM, discusses its regulatory standards (such as PM10 and PM2.5), and explains the significance of aerodynamic diameter and particle behavior in the atmosphere. The dialogues also touch upon health implications and the evolving understanding of particulate matter dynamics.
Detailed
Detailed Summary
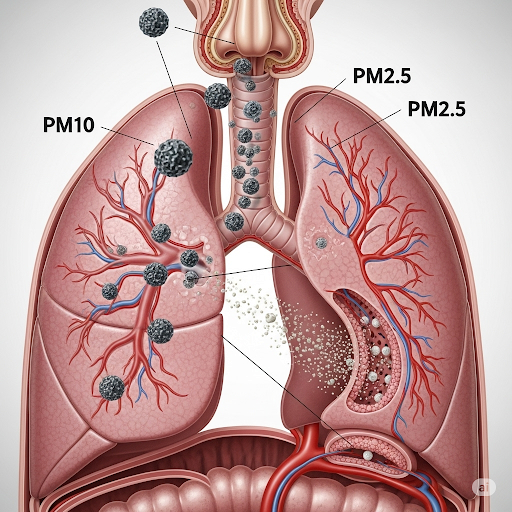
This section delves into the crucial role of Particulate Matter (PM) in assessing air quality. The primary focus is to define PM and understand its classifications, particularly PM10 and PM2.5, which correspond to particles less than 10 microns and 2.5 microns in aerodynamic diameter, respectively.
The teacher explains how PM is characterized using the concept of aerodynamic diameter, which considers the settling velocities of particles in fluid dynamics. The significance of PM sizes lies in their potential health impacts; smaller particles have a higher likelihood of reaching deep into the lungs due to their ability to bypass the respiratory system's filtration mechanisms, making them particularly concerning for public health.
Additionally, the discussion includes various generation mechanisms of particulate matter, indicating their origin from combustion processes and atmospheric reactions, and highlights the importance of regulatory references from agencies like the US EPA.
The section concludes with an acknowledgment that the understanding of PM is evolving and aligned with emerging health studies, requiring ongoing research and regulations to keep pace with new findings.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Particulate Matter (PM)
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Particulate matter is one of the most commonly used parameters for air quality. People quote particulate matter, PM this and PM that. So what is the definition of this? According to specifications from regulatory agencies like the US EPA, PM refers to any particles suspended in the air, which are categorized based on their size, primarily PM10 and PM2.5.
Detailed Explanation
Particulate Matter (PM) is an important measure of air quality. Regulatory agencies like the US EPA define PM as tiny particles that float in our air. The sizes of these particles are crucial; they are categorized into two main groups: PM10 (particles smaller than 10 micrometers) and PM2.5 (particles smaller than 2.5 micrometers). The size of these particles impacts how they behave in the environment and how they affect human health.
Examples & Analogies
Imagine a crowded room filled with people of different heights. The taller individuals represent PM10, while the shorter individuals represent PM2.5. Just as shorter individuals can fit under doors and into tighter spaces more easily, PM2.5 particles can penetrate deeper into our lungs compared to PM10 particles.
Understanding Particle Size
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The numbers in PM10 and PM2.5 refer to the size of the particles in micrometers. PM10 includes all particles that have a diameter less than 10 micrometers, while PM2.5 refers to those less than 2.5 micrometers. This is significant for understanding how particles behave in the air and their potential health effects.
Detailed Explanation
When referring to PM10 and PM2.5, the number serves as a threshold for particle size: 10 and 2.5 micrometers respectively. These sizes are critical, as smaller particles can remain suspended in the air longer, and they have a greater chance of entering the respiratory system when inhaled. The concept of aerodynamic diameter means that the behavior of particles, especially how they settle in the atmosphere, is influenced not only by size but also by their shape and density.
Examples & Analogies
Think of a basketball (PM10) and a grape (PM2.5). The basketball is large and more likely to be knocked out of the air by a breeze, while the grape is small enough to float with the wind and stay airborne longer. Likewise, PM2.5 can bypass filters in our noses and make it into our lungs, whereas many PM10 particles may get caught and filtered out.
Aerodynamic Diameter and Settling Velocity
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The aerodynamic diameter is defined as the diameter of an equivalent spherical particle that has the same settling velocity in a fluid, typically a density of 1 gram per cubic centimeter. Settling velocity is the constant speed a particle reaches when the downward force of gravity is balanced by the drag force of the fluid.
Detailed Explanation
Aerodynamic diameter is crucial because it helps predict how particles behave when suspended in air. This diameter is based on how quickly a particle will settle under gravity compared to a particle of a known size and density. Settling velocity indicates that larger, denser particles will fall faster than smaller particles under the same conditions.
Examples & Analogies
Picture dropping a feather and a stone simultaneously. The stone (larger mass and density) falls to the ground more quickly than the feather, which is lighter and has a larger surface area relative to its weight, resulting in more air resistance. Similarly, in air pollution, larger particles fall faster, while smaller particles may remain suspended longer, affecting how they are inhaled.
Health Implications of Size
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Because of their size, PM2.5 particles can penetrate deeper into the lungs compared to PM10 particles, which tend to get trapped in the upper respiratory tract. This distinction is vital since smaller particles are associated with more severe health risks.
Detailed Explanation
The difference between PM10 and PM2.5 is significant in terms of health because PM2.5 particles can bypass the body’s natural defenses, reaching the lungs and even entering the bloodstream. Research indicates that PM2.5 is linked to various health problems, including respiratory and cardiovascular diseases.
Examples & Analogies
Imagine trying to hide a marble (PM10) under a couch; it's difficult because it's too large to fit through tight spaces. Now imagine a grain of sand (PM2.5); it easily slips through the cracks and goes unnoticed. Just like the grain of sand can enter spaces that the marble cannot, PM2.5 can infiltrate deeper parts of our lungs, leading to more severe health impacts.
Particle Behavior in the Respiratory System
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Larger particles have more inertia and are less capable of navigating through the twists and turns of the respiratory tract, leading to deposition at various points throughout it, while smaller particles are more likely to reach the lungs and alveolar sacs.
Detailed Explanation
When we inhale, air that contains particles travels through a complex path in our respiratory system. Larger particles tend to get trapped along this path due to their momentum and inertia, while smaller particles are able to travel further into the lungs. This means that understanding particle behavior is essential for assessing air quality and the potential impact on health.
Examples & Analogies
Consider a wide truck (larger particles) navigating through winding streets versus a small car (smaller particles) moving easily through narrow alleys. The truck may get stuck or have to stop at every turn, while the car can maneuver freely, representing how larger particles often get trapped in our upper respiratory pathways while smaller particles can reach deeper into the lungs.
Impactions and Deposition Mechanisms
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The mechanisms through which particles deposit in the respiratory system include inertial impaction, interception, Brownian motion, gravitational settling, and electrostatic attraction. Each of these plays a role in how particles interact with the human body when inhaled.
Detailed Explanation
Particles can settle or get caught in the respiratory tract through various mechanisms. Inertial impaction occurs when larger particles can’t follow the air streamlines around an obstacle and hit it directly. Interception happens when particles come close to a surface and stick to it. Smaller particles may follow air flows due to their low inertia but can still adhere due to their random motion (Brownian motion). Understanding these mechanisms helps in assessing how particles might impact health.
Examples & Analogies
Think of how some balls bounce off a wall, while a soft sponge may gently stick to it. Larger particles (like the balls) can bounce off or be deflected, while smaller particles (like the sponge) might adhere to the surfaces in the respiratory tract, emphasizing the varied interactions depending on size.
Key Concepts
-
PM10: Particulate matter with a diameter of less than 10 microns.
-
PM2.5: Particulate matter with a diameter of less than 2.5 microns.
-
Aerodynamic Diameter: The size of a particle as defined by its behavior in air.
-
Settling Velocity: How quickly a particle settles in a fluid due to gravity.
-
Health Risks: Smaller particles (like PM2.5) pose a greater risk to health as they can travel deeper into the lungs.
Examples & Applications
Example of PM: Dust from construction sites and smoke from industrial emissions.
Health risks from PM2.5: Increased rates of asthma and other respiratory illnesses due to fine particulate matter.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Dust and smoke, big and small, PM makes us breathe it all.
Stories
Once upon a time, tiny PMs traveled through the air, some got trapped in noses, but the sneaky PM2.5 made its way deep into lungs, causing chaos!
Memory Tools
For remembering PM sizes: 'PM10, not so fine; PM2.5, health's decline!'
Acronyms
To recall PM types, remember 'P and M - Part & Micro!'
Flash Cards
Glossary
- Particulate Matter (PM)
A mixture of solid particles and liquid droplets found in the air.
- PM10
Particulate matter with a diameter of less than 10 microns.
- PM2.5
Particulate matter with a diameter of less than 2.5 microns.
- Aerodynamic Diameter
The diameter of an equivalent spherical particle that has the same settling velocity as the actual particle.
- Settling Velocity
The constant speed at which a particle falls through a fluid due to gravity.
Reference links
Supplementary resources to enhance your learning experience.
