Environmental Implications
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Partitioning Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll discuss how partitioning constants help us understand the fate of contaminants in the environment. Can anyone explain what we mean by partitioning?

I think it's about how different substances distribute among water, solids, and air.

Exactly! Partitioning is crucial in determining where a contaminant ends up. This leads us to the concept of equilibrium. What do you think equilibrium means in this context?

Is it when the concentration of the contaminant is the same in all phases?

Great point! Equilibrium means the concentrations stabilize across different phases. Remember, we often deal with equations that calculate how much contaminant moves into water or solids. This will be highlighted with mass balance equations.
Calculations Involved in Partitioning
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive into the calculations! Consider a system with 100 kilograms of a contaminant, A, added to a water and solid mixture. Why is it important to know the mass of A?

Is it because we need to understand how much of it ends up in water versus solid?

Precisely! We want to perform a mass balance. What can we say about A's distribution between water and solids at equilibrium?

We could use equations to determine the concentration in both phases, right?

Absolutely! One key equation we'll be working with is the mass balance: mass of A equals the sum of A in water and A in solids. Keeping track of units in these calculations is crucial!
Understanding Moisture Content and Its Implications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Another important factor is moisture content in solids. Can someone define moisture content and its relevance?

Moisture content is the mass of water relative to the mass of solids, but it can be calculated two ways: wet solid and dry solid basis.

Correct! It affects our calculations. Why do you think using a dry basis might be more convenient?

Using dry solids ensures we have a constant reference and avoid variations in water weight.

Well said! When analyzing contamination, knowing the correct moisture content helps us accurately assess potential environmental impacts.
Practical Applications and Environmental Relevance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s apply what we've learned! Suppose a chemical spill occurs in a river. How would partitioning constants help us understand the consequences?

They would help us estimate how much of the chemical dissolves in the water versus settling in sediments.

Exactly! By understanding the worst-case scenario, we can approach cleanup strategies effectively. What’s a worst-case scenario?

It’s when we assume the highest possible concentration of pollutants in water and solids to prepare for the contamination effects.

Great point! Preparing for worst-case helps in making informed management decisions in environmental response.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses how partitioning constants guide our understanding of how chemicals distribute between soil, water, and air. It introduces key definitions and calculations related to contaminant fate transport, emphasizing the need for careful data and equilibrium considerations.
Detailed
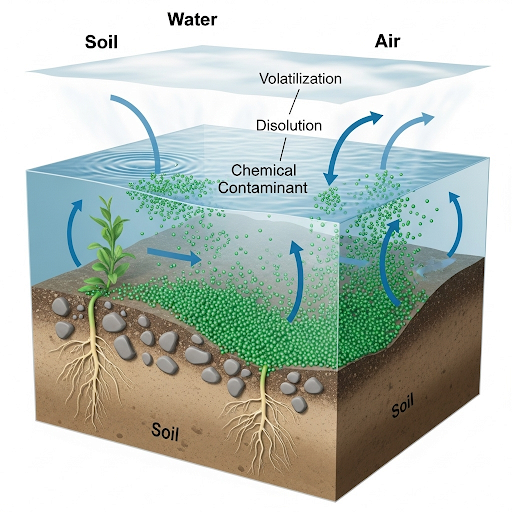
In this section, we explore the concept of soil-air partition constants and their relevance in environmental monitoring and analysis. The discussion begins with a simple closed system featuring both water and solids, into which a chemical contaminant is introduced. Key questions include how the chemical partitions between these phases and the implications of this distribution for environmental contamination. Several parameters are defined, including the volume of water, mass of solids, moisture content, and partition coefficients. The importance of mass balance and equilibrium in determining the fate of contaminants in the environment is emphasized, directing attention to the limitations of simple models and the significance of understanding multi-phase interactions (e.g., water, soil, air) over time. This analysis sets the foundation for making informed environmental assessments and management decisions.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Environmental Implications
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So we look at the application of partitioning, so the true application of partitioning constants that we looked at will become more obvious when we start doing transport. But for now, we will look at something very simple which when we will explain that why this is not relevant in its state the way in which we define it but it’s very useful in getting some basic information from contaminant fate transport point of view.
Detailed Explanation
This chunk introduces the concept of partitioning constants and their application in environmental studies. Partitioning constants help in understanding how pollutants, such as chemicals, distribute between different environmental compartments (like soil, water, and air). While partitioning may seem theoretical, it provides basic insights necessary for assessing how contaminants will behave in the environment as they spread or degrade over time.
Examples & Analogies
Think of partitioning like making a fruit salad. When you mix different fruits (like apples, oranges, and bananas), each fruit takes up a certain amount of space in the bowl, depending on how juicy or dense they are. Similarly, when pollutants enter the environment, they distribute themselves among soil, water, and air based on their characteristics, much like how different fruits have their own sizes and textures.
Defining the System
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So for example let’s take a very simple example that I have a system, so I am not going to use systems like soil sediments and all that because it’s very impractical. So, let’s say I have a system of a closed container which has some soil or sediments. Now let’s say it has some solids. This is similar to soil and sediments. And let’s say we have water, ok, we will start with these two systems first as of now then we will move on to the third one.
Detailed Explanation
In this part, the speaker sets up a simple hypothetical scenario to help understand how contaminants interact with the environment. A closed container is used as an example, which contains both solids (representing soil or sediments) and water. This clear setup allows focus on how a chemical might be introduced into the environment and how we can study its behavior.
Examples & Analogies
Imagine a small aquarium where you have both gravel (representing soil) and water. If you pour in some food coloring (the chemical), you can visually track how the color spreads through the water and interacts with the gravel over time. This experimentation helps you understand how pollutants might behave in a real pond or lake.
Chemical Addition and Partitioning Question
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Now, into this system I will add, let us say I will add 100 kilograms of some chemical A. So, what do we mean by adding 100 kilograms of A, is say there is the contamination, there is a pollution problem, so somebody dumps 100 kilograms of A into water system which contains water and solid into the system. So very straightforward problem but what we are going to look at some of the calculations that will do; So the question that we will ask is the following: How much of A will partition into water/solids? or other words what fraction of A will end up in water or the solids?
Detailed Explanation
This chunk introduces a real numerical example where 100 kilograms of a chemical is added to the system. The core analytical question involves understanding how much of that chemical (A) will settle into the water or the solid (soil/sediment) parts of the system. This highlights the importance of partitioning in environmental analysis - knowing what fraction of a contaminant resides in various components helps assess pollution impact.
Examples & Analogies
Picture someone adding a scoop of powdered drink mix (like lemonade) into a glass of water. If you stir it well, not all the powder dissolves immediately; some might still settle at the bottom. By knowing how much dissolves versus how much settles, we can understand the drink's overall flavor, just as scientists need to understand how much of a pollutant dissolves in water versus remains in the sediments.
Understanding Moisture Content in Solids
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So I have to give you what is the porosity of the solids with its water content, so ‘theta’ which is the ‘moisture content’ let us say it is 0.5. So, the definition of moisture content we have to be very careful, there are different definitions of moisture content people use...
Detailed Explanation
Moisture content is a key factor in understanding how contaminants behave, especially in soils. It indicates how much water is present in the soils relative to solids. This moisture affects not only the physical properties of the soil but also how chemicals behave when introduced to the system. Here, moisture content is expressed as a ratio – and it’s crucial that definitions are consistent to avoid miscalculations in environmental assessments.
Examples & Analogies
Consider a sponge. A dry sponge holds minimal water, but if you soak it, it becomes saturated and may even drip. If you measure how full it is compared to when it was dry, that’s like measuring moisture content in soil! Just like a sponge's water content changes how it feels and works, moisture in soil influences how chemicals might spread and be absorbed by plants or microbes.
Mass Balance in the Environment
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So we write mass balance, what is there initially, we are doing mass balance of A in the system, so the mass balance of A in the system is initial...
Detailed Explanation
Mass balance is a fundamental concept in environmental science that ensures the total amount of a substance before and after changes remain equal, barring any losses. In the context of the segment, the focus is on how the initially added chemical A divides between water and solids within the closed system. Understanding mass balance helps predict potential environmental impacts.
Examples & Analogies
Think about filling a balloon with water. You fill it up all the way, but water doesn’t magically disappear! You can take measurements before and after the balloon is full to see that all the water is inside it. In environmental contexts, just as with the balloon, we study what happens to each drop of chemical when introduced to the environment.
Concentration and Solubility Relationship
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Now we have to check one thing 0.05 kilogram per metre cubed multiplied by 10 raised to 6 milligrams per kilogram into 10 raised to 3 litres per metre cubed.
Detailed Explanation
Solubility indicates the maximum amount of a substance that can dissolve in a particular solvent at a given time. In checks like the concentration calculation here, if the calculated concentration exceeds solubility, it signals a miscalculation or the presence of undissolved material. Environmental scientists must monitor these interactions carefully to understand real contaminant behavior.
Examples & Analogies
Imagine a glass of water where you keep adding sugar. Initially, it dissolves completely, but after a point, some sugar starts to settle at the bottom because it can't dissolve anymore – that’s the solubility limit! In environmental terms, exceeding solubility means that not all pollutants can stay dissolved, leading to potential issues in contamination assessments.
Worst-Case Scenarios in Environmental Management
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So then we can plan for worst case scenario, for example in the earlier problem I know that lot it is still sitting as pure phase it has not gone into the soil.
Detailed Explanation
Exploring worst-case scenarios helps environmental managers devise effective mitigation strategies. By understanding the maximum potential impact of a contaminant — how much of it may remain in pure form without seeping into the soil — professionals can better prepare for responses to pollution events. This proactive planning is crucial for effective environmental management.
Examples & Analogies
Consider the safety drills schools perform for emergencies like fires. The drills help everyone practice and prepare for the worst-case scenario, ensuring that they know how to react and stay safe. Similarly, when dealing with environmental hazards, preparing for the worst possible pollution situation ensures that swift and effective action can be taken.
Key Concepts
-
Partitioning Constants: Determine how much contaminant divides between water and solids.
-
Mass Balance: Ensures all components of the contaminant are accounted for in a system.
-
Equilibrium: Represents a stable concentration of a contaminant across phases.
-
Moisture Content: Influences calculations related to the fate of contaminants in different mediums.
Examples & Applications
Example 1: If a pollutant is present at 100 kg, and it partitions into 10 kg in water and 90 kg in sediments, understanding this distribution is critical for mitigation efforts.
Example 2: If the moisture content of soil is 0.5 and affects pharmaceutical contaminant absorption, calculations must reflect this to evaluate environmental risk.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
For every chemical spill, remember the thrill, in solids and waters, a balance we fulfill!
Stories
Imagine a river with a giant chemical spill. The team rushes in to measure – how much in water? How much in soil? They work to understand, looking for a balance, striving for equilibrium amidst the chaos.
Memory Tools
M.E.C.: Mass, Equilibrium, Concentration – the key elements in managing contaminants.
Acronyms
P.E.A.C.E.
Partitioning Estimation And Concentration Equilibrium – a guide for students to remember their calculations.
Flash Cards
Glossary
- Partitioning Constant
A value that describes how a contaminant divides between different phases (e.g., soil and water).
- Moisture Content
The mass of water in a soil sample relative to the mass of solids, often expressed as a ratio.
- Mass Balance
A calculation that accounts for all mass inputs and outputs in a system to ensure conservation of mass.
- Equilibrium
A state in which the concentrations of a contaminant in different phases remain constant over time.
- Concentration
The amount of a substance in a given volume, often expressed as mass per unit volume (e.g., mg/L).
Reference links
Supplementary resources to enhance your learning experience.
