System Definition for Partitioning
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Partitioning Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today, we will discuss the concept of partitioning constants and their significance in environmental science. Who can tell me what partitioning means in this context?

It’s about how a chemical distributes between different phases, like water and soil, right?

Exactly! Partitioning helps us understand where contaminants go in the environment. Can anyone think of why that’s important?

It’s important for tracking pollution and figuring out how to clean it up!

Good point! Now, let’s define what we mean by our system. We typically analyze a closed container with soil and water. Let’s say we add a contaminant. Does anyone remember how we start with the calculations?

We need to know the total mass added and then determine how much partitions into each phase.

Right! Always start with a mass balance: Total mass equals mass in water plus mass in solids. This is foundational.
Understanding Moisture Content
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s discuss moisture content. Can someone explain how it is defined?

Is it the mass of water compared to the mass of wet solids?

That’s one way! But remember, it can also be based on dry solids. Why do you think this distinction is crucial?

It affects calculations when trying to assess how much water is actually in the system.

Exactly! This can impact mass balance significantly. Be careful with these definitions since they can lead to different results. Can anyone recall what moisture content we used in our example?

I think it was 0.5 or 50% based on the wet solids!

Great memory! Understanding these percentages is key to effective contaminant management.
Chemical Properties and Calculations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s analyze the chemical properties we need. What is the aqueous solubility of chemical A in our example?

It was 1.0 milligrams per litre!

Correct! And how does that tie into our partitioning constant Koc, which was defined as 4.0 in logarithmic form?

I remember Koc tells us how much the chemical prefers to stay in solids versus water!

Yes! This ratio is essential for predicting contaminant behavior. So if we add too much chemical, what might happen?

The concentration could exceed solubility and lead to solid phase being present.

Exactly! That’s where our calculations come into play to avoid errors in our assessments. Always check your results against solubility.
Mass Balancing in Environmental Systems
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s put everything together using our mass balance. If we added 100 kilograms of chemical A, how do we ensure our balance holds?

We write 100 kilograms equals mass in water plus mass in solids and residual A!

Perfect! And when we calculate, if we find our concentration in water exceeds solubility, what should we assume?

Some A remains in solid form because it can’t dissolve beyond its solubility!

Exactly. That’s critical for our understanding of contamination in sediment and its potential impacts. Can anyone summarize what we’ve learned?

We learned about partitioning constants, moisture definitions, and how to perform mass balances to track contaminants!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section delves into the definitions and calculations associated with partitioning constants in environmental contexts. It discusses mass balance, moisture content definitions, and chemical properties related to partitioning in soil and water systems, providing foundational knowledge for further analysis in environmental quality monitoring.
Detailed
System Definition for Partitioning
This section focuses on the concept of partitioning constants in environmental science, particularly their application in understanding the fate and transport of contaminants within soil and water systems. The professor outlines the importance of defining the system correctly, specifically in terms of the materials involved and their properties, such as moisture content and chemical characteristics.
To illustrate these principles, an example is provided involving a closed container containing soil, water, and a contaminant (chemical A). Here, initial mass balance principles are introduced, showcasing how a given mass of chemical partitions into different phases, namely water and solids.
Key parameters such as the volume of water, mass of solids, moisture content, and chemical properties like the partition constant (Koc) and aqueous solubility are defined and used for calculations. Special attention is given to the definitions of moisture content and the different ways it can be expressed (wet versus dry basis).
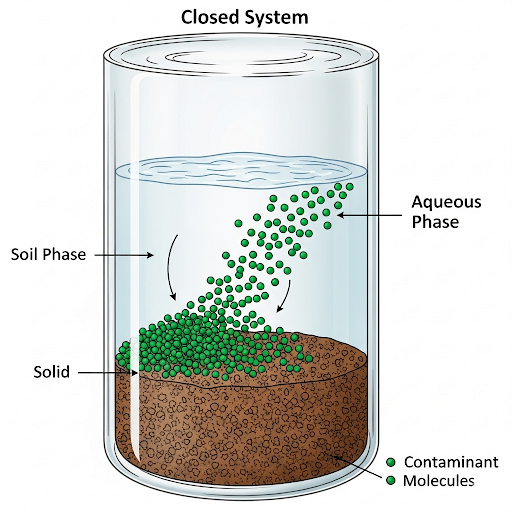
The section emphasizes the significance of accurate calculations in tracking contaminant distribution and highlights potential mass balance errors, thereby establishing foundational knowledge crucial for environmental quality monitoring and analysis.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Partitioning in Environmental Systems
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, we look at the application of partitioning, so the true application of partitioning constants that we looked at will become more obvious when we start doing transport. But for now, we will look at something very simple which when we will explain that why this is not relevant in its state the way in which we define it but it’s very useful in getting some basic information from contaminant fate transport point of view.
Detailed Explanation
The introduction sets the stage for understanding how partitioning constants apply to environmental systems, particularly in monitoring contaminants. It explains that while the definition has limitations, it serves as a foundational tool for understanding contaminant behavior in different environmental phases (like water or soil).
Examples & Analogies
Imagine you are trying to understand how food ingredients mix when cooking. Each ingredient behaves differently based on its characteristics (like how it dissolves or remains separate). Similarly, partitioning helps us understand how contaminants behave in water and soil.
Defining the System
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So for example let’s take a very simple example that I have a system, so I am not going to use systems like soil sediments and all that because it’s very impractical. So, let’s say I have a system of a closed container which has some soil or sediments. Now let’s say it has some solids. This is similar to soil and sediments. And let’s say we have water, ok, we will start with these two systems first as of now then we will move on to the third one, ok.
Detailed Explanation
This part explains how to define the system for analysis. The example used is a closed container filled with soil (or solids) and water. It simplifies the analysis, as real-world systems can be complicated due to various factors like movement and interaction. It helps students visualize how contaminants are studied within a controlled framework.
Examples & Analogies
Think of a fish tank where you have gravel (representing soil) and water. Just like you observe how fish interact with the water, we observe how chemicals move in the water and gravels in the container.
Adding Chemical A to the System
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Now, into this system I will add, let us say I will add 100 kilograms of some chemical A. So, what do we mean by adding 100 kilograms of A, is say there is the contamination, there is a pollution problem, so somebody dumps 100 kilograms of A into water system which contains water and solid into the system.
Detailed Explanation
In this part, the scenario shifts to introducing a chemical into the system. Adding 100 kg of a contaminant (A) simulates pollution in the environment. Students learn that analyzing how this chemical distributes between the water and the soil helps us understand its fate and transport.
Examples & Analogies
Imagine someone pours a large amount of sugar into water. The sugar will dissolve and spread throughout the liquid, but if there's too much sugar for the water to hold, some will remain at the bottom. This illustrates how contaminants behave when introduced into an environmental system.
Understanding Partitioning of Chemical A
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, the question that we will ask is the following: How much of A will partition into water/solids? or other words what fraction of A will end up in water or the solids?
Detailed Explanation
This section introduces key questions regarding the distribution of chemical A, which is essential for understanding its environmental impact. Understanding partitioning means identifying how much of the contaminant will end up in the water versus the soil, which is crucial for risk assessment and remediation efforts.
Examples & Analogies
Consider making a salad, where each ingredient has a different amount of dressing. Just like the dressing sits on some ingredients but is absorbed by others, a contaminant will distribute differently based on its properties and the environmental conditions.
Mass Balance Considerations
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So we write mass balance, what is there initially, we are doing mass balance of A in the system, so the mass balance of A in the system is initial and what we did in the calculation in the last class for the partitioning, we are doing partitioning.
Detailed Explanation
The concept of mass balance is introduced here, emphasizing that the initial mass of chemical A added must equal the total mass of A after partitioning into the water and soil phases. This understanding is foundational in environmental science as it ensures that all components of the system are accounted for.
Examples & Analogies
Think about baking a cake where every ingredient must be measured precisely. If you add too much flour or sugar, the recipe will not turn out right. In environmental science, we must account for every part of the system just like baking.
Understanding Moisture Content
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, I have to give you what is the porosity of the solids with its water content, so ‘theta’ which is the ‘moisture content’ let us say it is 0.5.
Detailed Explanation
Moisture content is a key factor in determining how much water is retained in soils. Here, it is defined in terms of the ratio of mass of water to mass of solids. This information is critical as it affects how contaminants interact with the soil and water in the system.
Examples & Analogies
Imagine a sponge soaked in water. The amount of water the sponge can hold compared to its dry state is like moisture content. The more water it can retain, the better it can impact the surrounding systems, like releasing chemicals into the environment.
Calculating Chemical Properties
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The other data that I have is pertaining to A, chemical, the log K oc of A is 4.0 let me give you that, the aqueous solubility of A let say is 1.0 milligrams per litre.
Detailed Explanation
This part provides key chemical properties such as log K_oc (a measure of how a chemical partitions between water and organic carbon) and solubility. These values are essential for accurately calculating how chemical A behaves in environmental systems.
Examples & Analogies
Think of how some drinks are sweetened. If a drink can only hold a certain amount of sugar (solubility), adding too much will just leave sugar at the bottom. This is similar to understanding how much chemical A can dissolve in water versus what might remain in the soil.
Key Concepts
-
Partitioning: The process of how chemicals distribute in different environmental phases.
-
Moisture Content: Important for understanding chamber water availability in solid materials.
-
Koc: Critical for understanding the environmental fate of organic chemicals.
-
Mass Balance: Essential for ensuring total mass conservation in environmental assessments.
Examples & Applications
If 100 kg of chemical A is added to a system while maintaining a total mass balance, the partitioning will need to be computed to see how much remains undissolved based on solubility.
Moisture content might be expressed as a ratio of wet solids, affecting the total mass measurement in calculations.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In soils, our chemicals divide, between water and solids, they gently abide.
Stories
Imagine a chemical party where A wants to mingle with water but influencers like Koc decide who stays where, leading to a balanced mix.
Memory Tools
P-M-M: Partitioning means Mass Balance & Moisture - Keep these three in mind when assessing!
Acronyms
P.E.R.S.
Partitioning
Equilibrium
Ratio
Solubility - Remember this for chemical distributions!
Flash Cards
Glossary
- Partitioning
The distribution of a chemical between different phases, such as solid and liquid.
- Moisture Content
The ratio of mass of water to mass of solids, expressed on a wet or dry basis.
- Partition Constant (Koc)
A value indicating the tendency of a chemical to partition between organic carbon in solids and water.
- Aqueous Solubility
The maximum amount of a substance that can dissolve in water at a given temperature and pressure.
- Mass Balance
A principle stating that the total mass entering a system must equal the total mass leaving the system under steady state.
Reference links
Supplementary resources to enhance your learning experience.
