Detailed Example of Mass Spectrum Analysis
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Spectrometry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today, we are going to learn about mass spectrometry. Can anyone tell me what mass spectrometry is?

Isn't it a method to determine the mass of different molecules?

Exactly! Mass spectrometry helps us measure the mass-to-charge ratio of ions, which is key to identifying compounds. Remember the acronym M/S, for MassSpectrometry! Let's dive deeper. What happens during ionization?

That's when the sample gets ionized, right?

Right! When the sample is ionized, it breaks into fragments. This fragmentation is essential for analysis. Can anyone think of a reason why we need these fragments?

To distinguish between different compounds?

Exactly! These fragments carry specific mass-to-charge ratios, allowing us to identify and quantify the original compound.

In summary, mass spectrometry is crucial for analyzing substances because it measures mass-to-charge ratios of ionized fragments formed during ionization.
Mass Analyzers and Detectors
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss mass analyzers, particularly the quadrupole mass analyzer. What do you think those four rods do?

They filter ionized fragments based on their mass?

Correct! These rods create an electric field that allows certain mass-to-charge ratios to pass through while blocking others. This selective process is a key part of how we gather data. Can someone articulate what happens next?

The allowed fragments reach the detector, where their intensity is measured.

Spot on! Each fragment's intensity helps reconstruct a mass spectrum. So, how do we use this spectrum for identification?

We compare it to a library of known spectra!

Exactly! This library comparison is vital. In summary, quadrupoles filter fragments based on their m/z values, allowing us to gather data for compound identification.
Interpreting Mass Spectra
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s interpret mass spectra now! What does the x-axis generally represent?

The mass-to-charge ratio, M/Z?

Yes, and what about the y-axis?

That's the intensity of the fragments.

Exactly! Now, if we look at a spectrum with two prominent peaks at 91 and 106, how would we proceed?

We could compare those peaks to known spectra in a library.

Great! This helps confirm the identity of a compound by checking against known spectra. Remember to look for similarities and differences!

To conclude our session, understanding how to interpret mass spectra is crucial for accurately identifying chemical compounds.
Impact of Mass Spectrometry in Environmental Analysis
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s explore how mass spectrometry impacts environmental monitoring. Why do you think it’s crucial?

It helps identify pollutants and contaminants in samples!

Exactly! By analyzing polluted samples, we can detect harmful substances effectively. Can anyone give a specific example?

Maybe when checking water quality for pesticides?

Spot on! Pesticides can be effectively monitored using mass spectrometry. This empowers us to take necessary actions to protect environmental health. Remember, mass spectrometry provides vital information for maintaining environmental quality.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section elaborates on mass spectrometry as an analytical technique that utilizes ionization and fragmentation of organic compounds to analyze their mass-to-charge ratios. It emphasizes the role of mass analyzers, particularly quadrupoles, in the separation and identification of molecular fragments, and discusses the importance of library comparisons for compound identification.
Detailed
Detailed Example of Mass Spectrum Analysis
Mass spectrometry (MS) is a powerful analytical technique used primarily for identifying and quantifying chemical substances in various fields, including environmental monitoring. In the mass spectrometer, a sample enters through an ionization chamber where it is bombarded with moderate energy electrons, resulting in the ionization and fragmentation of the organic molecules. These fragments are then analyzed based on their mass-to-charge ratios (m/z) using devices known as mass analyzers.
One common type of mass analyzer is the quadrupole. It consists of four rods, functioning like magnets, that selectively filter ionized fragments according to specific m/z ratios. Through a rapid cycling process, various fragments are allowed to pass through to the detector at different time intervals, effectively reconstituting the information into a mass spectrum, which serves as a unique signature for each compound.
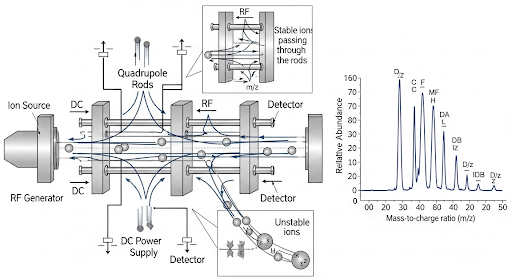
This mass spectr can then be compared to a library of standard spectra for identification purposes. For example, when analyzing a sample that contains benzene, the obtained spectrum can be cross-referenced with known spectral data to confirm or refute the identity of the compound. The fundamental principles of mass spectrometry are crucial for environmental quality monitoring since they provide detailed information on the composition and concentration of pollutants.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Mass Spectrometry
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Okay, so we are continuing our discussion on chromatography. So, we will discuss a little bit about mass spectrometer for organic analysis. In mass spectrometer detector, what happens is it is similar to the regular GC that has a GC column. The detectors in the mass spectrometer are significantly larger and more complex than those in regular gas chromatography techniques.
Detailed Explanation
In this section, we begin with an introduction to mass spectrometry as part of chromatographic analysis, highlighting its importance in analyzing organic compounds. Unlike a standard gas chromatograph (GC) that uses smaller detectors, mass spectrometry involves a complex detector that can analyze ions and their fragments. This sets the stage for understanding how mass spectrometry provides detailed information on the molecular composition of samples.
Examples & Analogies
Think of mass spectrometry like a high-tech detective agency for molecules. Just as a detective uses various tools to gather detailed evidence about a crime scene, mass spectrometry separates and analyzes fragments of molecules to 'detect' their structure and identity.
Ionization and Fragmentation
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In this trivializing theory, mass spectrometry is a very complex field and involves the interaction with energy of different forms. In this case, energy is in the form of high-energy electrons. What essentially happens here is that there is a fragmentation that occurs to organic molecules as they come into the instrument. Each time a molecule fragments, the instrument measures the intensity of each fragment.
Detailed Explanation
This chunk explains the process of ionization and fragmentation of organic molecules under the influence of high-energy electrons. When organic molecules are introduced into the mass spectrometer, they are ionized and broken into smaller fragments. The device measures how much of each fragment exists, which is crucial for identifying the original molecule. This fragmentation is key because the resulting fragments can give substantial insights into the structure and properties of the original molecules.
Examples & Analogies
Imagine you have a piece of fruit, like an apple. If you slice it into smaller pieces, the pieces can provide information about the whole fruit, such as its flavor or texture. In the same way, when a molecule gets fragmented, those smaller pieces tell scientists about the larger, complex structure of the original molecule.
Mass Analyzers and Detection
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The mass spectrometer instrument has a device called a mass analyzer. The sample coming from the GC flows into this ionization device followed by the mass analyzer, which subsequently separates the fragments based on their mass-to-charge ratios. Various types of mass analyzers exist, but one common type is the quadrupole.
Detailed Explanation
This section introduces mass analyzers, specifically focusing on the quadrupole analyzer. The analyzer works by allowing only certain fragments (based on their mass-to-charge ratio) to pass through for detection, similar to a filter. This selective passage of ions ensures that only relevant fragments are analyzed, which provides specific data about the chemical identity of the original sample.
Examples & Analogies
Imagine a bouncer at a club who only lets people in based on specific criteria. In this analogy, the quadrupole mass analyzer acts like that bouncer, only allowing certain 'fragments' (molecules) that match specific criteria (mass-to-charge ratio) to reach the detectors while filtering out the others.
Data Acquisition and Analysis
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The detector allows the mass analyzer to analyze each fragment, gathering intensity data over time. As compounds exit the GC, the mass analyzer scans through different mass-to-charge ratios rapidly, creating a mass spectrum that represents distinct peaks corresponding to the analyzed substances.
Detailed Explanation
This chunk details how the mass spectra are acquired and analyzed. As compounds elute from the GC, the mass analyzer scans through the mass-to-charge ratios, quickly gathering data on each fragment's intensity. This forms the mass spectrum, where each peak corresponds to different fragments of the compound, providing critical information that helps identify and quantify the components of the original mixture.
Examples & Analogies
Consider this process like a concert where different musical instruments play at different times. The mass spectrum acts as the concert program, with each peak representing a unique instrument's performance. By looking at the program, you can identify which instruments (fragments) were involved in the performance (the original molecule).
Identifying and Verifying Compounds
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To identify a compound, one must compare the obtained mass spectrum against a library of known spectra. This comparison process allows researchers to check for matches, effectively determining the identity of the compound based on similarities in the spectral signatures.
Detailed Explanation
This section concludes with how to identify compounds using mass spectra. By comparing the experimental mass spectrum to a database of known spectra, researchers can verify the identity of a compound. This library comparison is essential in affirming whether the obtained spectrum matches a known compound, streamlining identification in various analytical settings.
Examples & Analogies
Think of identifying a compound like matching fingerprints in a police database. Just as investigators use a fingerprint library to find a suspect's identity, scientists use a mass spectrum library to confirm the identity of a chemical based on its fragmentation pattern.
Key Concepts
-
Ionization: The conversion of molecules into ions for analysis.
-
Mass Analyzer: Device that separates ions by mass-to-charge ratio.
-
Quadrupole: A common type of mass analyzer using four rods for filtration.
-
Mass Spectrum: Graphical display of intensity versus mass-to-charge ratio.
Examples & Applications
Mass spectrometry can identify pollutants in water samples, such as pesticides.
Analyzing a mass spectrum can confirm the presence of benzene by matching its fragments against known standards.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In mass spec, fragments go zap, filtered by rods, in a quick map.
Stories
Imagine a wizard who can turn compounds into fragments, showcasing their magical identities through a spectacular mass spectrum.
Memory Tools
I-F-M-S: Ionization, Fragmentation, Mass Analyzer, Spectrum.
Acronyms
M/S
Mass & Spectrometry for identifying the mystery behind compounds.
Flash Cards
Glossary
- Mass Spectrometry
An analytical technique used to measure the mass-to-charge ratio of ions, allowing the identification of compounds.
- Ionization
The process of converting a molecule into ions, facilitating its analysis in mass spectrometry.
- Mass Analyzer
A device used in mass spectrometry that separates ions according to their mass-to-charge ratio.
- Quadrupole
A type of mass analyzer that uses four rods to filter ions based on their mass-to-charge ratios.
- Mass Spectrum
A graphical representation showing the distribution of ion intensities across different mass-to-charge ratios (m/z).
Reference links
Supplementary resources to enhance your learning experience.
