Mobile Phase in Liquid Chromatography
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mobile Phase
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore the mobile phase in liquid chromatography. Can anyone tell me what you think the mobile phase is?

Is it the liquid that carries the sample through the system?

Exactly! The mobile phase is indeed the solvent or mixture of solvents that transports the sample through the chromatographic column.

Why is it important to have a good choice of mobile phase?

Great question! The choice of mobile phase affects separation efficiency and is fundamental in determining how well different compounds interact with the stationary phase.

Can we change the mobile phase during the run?

Yes! Unlike gas chromatography, LC allows you to adjust solvent composition over time to optimize separation.

That sounds interesting! How does that work in practice?

It works like this: if you start with a low concentration of solvent A and gradually increase it, you can change the polarity, which can help separate compounds more effectively.

To summarize, the mobile phase is crucial in LC, and its flexibility allows for enhanced separation techniques.
Temperature Control in Liquid Chromatography
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about temperature in liquid chromatography. Why do you think temperature management is vital?

Is it to help the liquid flow better?

Exactly! Maintaining a consistent temperature prevents bubbles caused by vaporization, which can disrupt flow.

What happens if those bubbles form?

Bubbles can cause pressure fluctuations and affect retention times, leading to inaccurate results.

What are typical temperature ranges in LC?

Generally, the temperature range maintained is between 25 to 40 degrees Celsius, which helps in consistent mobile phase behavior.

In summary, managing temperature during liquid chromatography is crucial to ensuring accurate and reliable separation.
Detectors in Liquid Chromatography
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss the detectors used in LC. Who can name some detectors that we might use?

I've heard of UV detectors; are there others?

Exactly! In addition to UV detectors, we have visible spectroscopy, refractive index detectors, and fluorescence detectors.

How do these detectors work?

For UV detectors, they measure the absorbance of light at specific wavelengths. Higher concentrations of a compound will result in higher absorbance readings.

Can you explain how absorbance relates to concentration?

Certainly! There is a direct relationship: as the concentration of a compound increases, the amount of light absorbed also increases, which can be quantified.

In summary, different detectors serve various functions, with UV and fluorescence being common due to their sensitivity and reliability.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores the mobile phase in liquid chromatography, comparing it with gas chromatography, and detailing the significance of solvent selection, temperature control, and detection methods. It emphasizes the flexibility of solvent mixtures and the careful management of conditions to prevent issues like vaporization and pressure fluctuations.
Detailed
In this section, we delve into the role of the mobile phase in liquid chromatography (LC), a critical parameter that defines the effectiveness of this analysis technique. Unlike gas chromatography (GC), where the sample is vaporized for analysis, LC processes liquid samples without the complications of vaporization. The mobile phase, comprising a single solvent or a mixture, plays a vital role in isolating compounds based on their interaction with the stationary phase. The section discusses how temperature in LC is carefully controlled to maintain flow without introducing vapor bubbles, which can disrupt the analytical process. In contrast to GC, where temperature is pivotal, LC allows for adjustments to solvent composition, enhancing separation capabilities based on polarity. Various detectors are employed, including UV, visible spectroscopy, refractive index, and fluorescence, to identify and quantify the separated compounds. The understanding of how mobile phase characteristics influence separation outcomes is essential for proficiently utilizing LC in analytical chemistry.
Youtube Videos


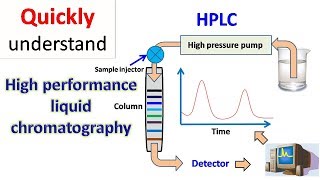







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding the Mobile Phase
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The mobile phase is a solvent; it can be a single solvent or a mixture of solvents.
Detailed Explanation
In liquid chromatography, the mobile phase refers to the solution that carries the sample through the column. This phase can either consist of just one type of solvent or a combination of different solvents. The choice of mobile phase is crucial because it affects how well the sample components are separated during the chromatography process. For example, in a simple setup, one might use pure water as the mobile phase, while in more complex applications, a mix of solvents like water and acetonitrile is preferred to enhance separation efficiency.
Examples & Analogies
Think of the mobile phase like a river flowing through a landscape. The river (mobile phase) carries various particles (sample components) along different paths depending on the characteristics of the water (solvent) and the land features (stationary phase). Just as certain terrains will slow down or speed up the movement of river particles, the choice of solvents will influence how different compounds in the mixture are separated as they flow through the chromatography column.
Temperature Control in Liquid Chromatography
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
You can change the temperature in gas chromatography, but this is not common in liquid chromatography due to the risk of bubbles forming.
Detailed Explanation
In gas chromatography, temperature changes can help to manipulate the vaporization of samples for better separation. However, in liquid chromatography, temperature is generally kept constant because altering it can cause the liquid solvents to boil, leading to the formation of bubbles. These bubbles can obstruct the flow of the mobile phase, causing inconsistencies in the results. Therefore, liquid chromatography systems typically maintain a constant temperature, usually within a range of 25 to 40 degrees Celsius, to ensure stable operation and consistent retention times for accurate analysis.
Examples & Analogies
Imagine trying to pour syrup from a bottle. If the syrup is too hot, it might bubble and spill, creating a messy situation. Similarly, if the temperature in liquid chromatography is not controlled, it can lead to bubbles that interrupt the careful flow of liquid and lead to analysis errors.
Changing Solvent Composition
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
You can change the solvent composition dynamically, which allows the adjustment of polarity during the process.
Detailed Explanation
One of the key advantages of liquid chromatography is the ability to dynamically adjust the mobile phase composition. By starting with one solvent and gradually increasing the concentration of another solvent, chromatographers can control the polarity of the mobile phase. This adjustment influences how the sample components partition between the mobile phase and the stationary phase within the column, ultimately affecting separation efficiency. For instance, starting with a low concentration of acetonitrile and gradually increasing it allows for better separation of compounds with different polarities.
Examples & Analogies
Consider baking a cake where the ingredients matter. You start with flour and gradually add sugar and eggs to get the perfect mixture. In the same way, changing the solvent composition in liquid chromatography ensures that the 'recipe' for separating compounds is just right, allowing for better results in identifying and quantifying substances.
Detectors in Liquid Chromatography
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Common detectors used in liquid chromatography include UV, visible spectroscopy, refractive index, and fluorescence.
Detailed Explanation
Detecting compounds in liquid chromatography is crucial for analyzing what has been separated in the column. Various types of detectors are employed, with some of the most common being UV-visible spectroscopy, refractive index detectors, and fluorescence detectors. UV and visible detectors are particularly important because they can identify compounds based on their absorbance of specific wavelengths of light. This allows scientists to determine both the presence and concentration of the analytes as they exit the chromatography column. Each detector type has its strengths and weaknesses, affecting its suitability for different types of analytes.
Examples & Analogies
Think of a detector like a librarian. Just as a librarian uses their knowledge to identify and locate books based on subject matter (UV-visible absorbance), references (refractive index), or special collections (fluorescence), the detectors help chemists identify and quantify the components of a mixture based on their unique properties.
Key Concepts
-
Importance of Mobile Phase: The mobile phase is crucial for transporting samples and affecting the separation process.
-
Temperature Control: Consistent temperature prevents vaporization and pressure fluctuations.
-
Detector Types: Various detectors, such as UV and fluorescence, are used for analyzing outcomes.
Examples & Applications
In liquid chromatography, a mixture containing acetonitrile and water is gradually adjusted to enhance separation for different compounds based on their polarity.
UV-Vis detectors measure the absorbance of a sample, allowing quantification based on concentration using Beer's Law.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In chromatography so fine, the mobile phase flows in line; keep the temp just right, to keep bubbles from sight.
Stories
Imagine a race where a liquid sample rides on a wave of its mobile phase, carefully managed temperature prevents bubbling troubles, ensuring a smooth victory across the chromatography line.
Memory Tools
MDR (Mobile phase, Detection, Retention time) - Remember the key factors in liquid chromatography.
Acronyms
LCMP (Liquid Chromatography Mobile Phase) - Always consider the mobile phase in liquid chromatography.
Flash Cards
Glossary
- Mobile Phase
The solvent or mixture of solvents that transports the sample through the chromatographic system.
- Liquid Chromatography (LC)
A technique for separating compounds in a mixture using liquid as the mobile phase.
- Gas Chromatography (GC)
A method of separating volatile substances through vaporization and column chromatography.
- Retention Time
The time a compound spends in the stationary phase before being detected.
- Detector
An instrument used to identify and quantify compounds as they elute from the chromatographic column.
- UVVis Spectroscopy
A method of detecting compounds based on their absorption of ultraviolet and visible light.
- Fluorescence Spectroscopy
A technique for detecting compounds by measuring the emitted light after excitation.
Reference links
Supplementary resources to enhance your learning experience.
