Introduction to Source Apportionment and Chemical Composition
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Source Apportionment
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing source apportionment, which is crucial for identifying the origins of particulate matter in our atmosphere. Can anyone explain why understanding the composition of particulate matter is important?

I think it helps to determine where the pollution is coming from, right?

Exactly! By figuring out where pollutants originate, we can address specific sources of air quality issues. What are some ways we can measure these sources?

Wouldn't mass distribution be one method?

Yes, but mass distribution alone isn’t enough. We need detailed chemical composition to get clearer signals about the sources.

So, we're looking for specific signatures for each source?

Correct! We call these signatures 'chemical fingerprints.' Let's summarize key concepts: source apportionment is vital for pollution management, and chemical composition provides the detailed information needed for effective analysis.
Measuring Particulate Matter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s delve into how we actually measure particulate matter. What do you think makes measuring ultra-fine particles challenging?

Are they too small to measure accurately?

Absolutely, they typically fall below 300 nanometers, which complicates measurement. What instruments can help us with this?

I remember the Differential Mobility Analyzer you mentioned last class!

Great memory! Can anyone describe how this analyzer functions?

It charges particles based on their size and uses a potential difference to sort them, right?

That's correct! We can also use a Scanning Mobility Particle Sizer to gather a more complete picture of the particle size distribution. Remember, accurate measurements are essential for understanding air quality.
Impact of Chemical Composition
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s shift our focus to why chemical composition matters. Could anyone provide an example of why knowing the composition of PM can be critical?

If we know that a certain composition comes from vehicles, we can introduce policies to reduce those emissions!

Exactly! That shows how actionable our findings can be. What happens if we misinterpret these measurements?

We might address the wrong sources of pollution or implement ineffective strategies.

Precisely. Misinterpretations can lead to wasted resources and unresolved health issues. Let’s recap: understanding chemical composition is vital for accurately managing air quality and addressing health risks.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the importance of chemical composition for accurate source apportionment of particulate matter. Various instruments and methods for measuring different particulate matter sizes are discussed, emphasizing the role these measurements play in identifying pollution sources and their impacts over different time spans.
Detailed
Introduction to Source Apportionment and Chemical Composition
This section provides an overview of source apportionment and its dependence on understanding chemical composition. Source apportionment refers to the attribution of particulate matter (PM) to its various sources, essential for addressing air quality issues. While mass distribution data can provide insights, they often lack the resolution needed to differentiate between multiple sources of atmospheric particles.
Chemical composition serves as a critical tool for overcoming these limitations by providing specific signatures for distinct sources of pollutants. However, caution is necessary due to differences in measuring aerodynamic diameter versus scattering diameter of particulates.
The section discusses ultra-fine particulate matter, which is particularly significant for emissions from vehicles, as many harmful particulates reside in this size range. Instrumentation used for measuring these particles, such as the Differential Mobility Analyzer and Scanning Mobility Particle Sizer, is introduced, highlighting the complexities involved in their measurement and data interpretation.
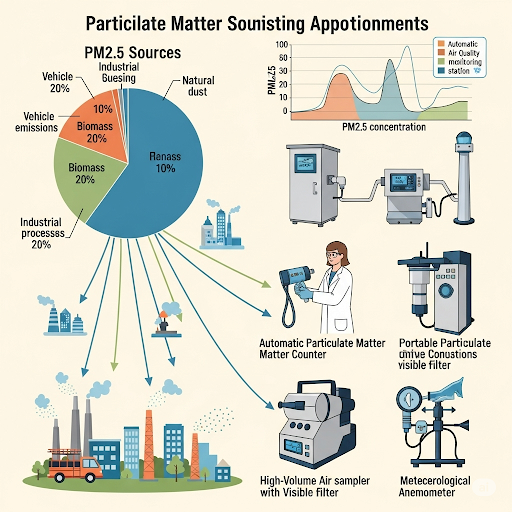
The importance of cost-effective methods and widespread measurement in air quality monitoring is emphasized, pointing out that understanding these dynamics is crucial for effective environmental policies and public health guidelines.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Importance of Chemical Composition
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Sometimes you want chemical composition, and you are getting a lot of material. So you are, again, you are looking at composition that composition will give you a lot of clues to where it is coming from just getting mass distribution is not enough.
Detailed Explanation
Chemical composition refers to the specific elements and compounds that make up a material. In the context of environmental science, understanding the chemical composition of pollutants is crucial. It helps researchers determine the sources of pollution. Simply knowing how much material is present (mass distribution) does not provide enough information, as multiple sources can contribute similar materials. Analyzing the chemical composition helps distinguish these sources.
Examples & Analogies
Think of it like a detective investigating a crime scene. If there are fingerprints everywhere, knowing how many fingerprints there are (mass distribution) is not enough. The detective needs to analyze the unique patterns of each fingerprint (chemical composition) to determine who was present at the scene.
Source Apportionment Explained
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
What we call a source apportionment, we have source of apportionment and you can do source apportionment as long as you get a very specific signal for a source.
Detailed Explanation
Source apportionment is the process of identifying the contributions of different sources to an observed concentration of pollutants. It requires a detailed understanding of the 'signals' or unique characteristics associated with each pollutant source. For instance, emissions from vehicles will have different chemical signatures compared to industrial emissions. By analyzing these specific signals, scientists can identify which sources are contributing to pollution levels.
Examples & Analogies
Imagine you are trying to figure out which fruit is responsible for the smell in your kitchen. Each fruit (like an apple, banana, or orange) has a distinct smell, like how pollutants from different sources have unique signals. By smelling each fruit, you can pinpoint the source of the odor in the kitchen, just like researchers pinpoint pollution sources using source apportionment.
Limitations of Optical Methods
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This optical method is not measuring aerodynamic diameter, this is measuring a scattering diameter.
Detailed Explanation
In environmental measurements, the diameter of particulate matter can be measured using various methods. However, optical methods may only assess the scattering diameter, which differs from the aerodynamic diameter. The aerodynamic diameter considers how particles behave when in motion through air, while scattering diameter pertains to how they scatter light. This distinction is essential when classifying particulate matter into categories like PM10 or PM2.5, as they have different health implications.
Examples & Analogies
Consider how a basketball (big and heavy) and a ping pong ball (small and lightweight) behave when thrown. The basketball's size gives it a substantial aerodynamic diameter, while the ping pong ball might scatter light differently due to its size and weight. When measuring air quality, understanding these differences helps determine which particles can penetrate deep into the lungs (health implications).
The Role of Ultra-Fine Particulate Matter
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ultra-fine particulate matter are particulate matter which are below 300 nanometers and below 500 nanometers in that size range. The reason this is important is a lot of emissions coming from vehicles, diesel analyzing particulate matter are in this size range.
Detailed Explanation
Ultra-fine particulate matter (UFP) refers to particles that are extremely small, typically less than 300 nanometers. These particles are especially concerning because many vehicles emit them during diesel combustion. Their tiny size allows them to penetrate deep into the lungs and even enter the bloodstream, potentially leading to various health problems. Understanding the sources and characteristics of UFP is crucial for health and environmental regulations.
Examples & Analogies
Think of ultra-fine particulate matter like tiny specks of dust that you can barely see. Just as these dust particles can settle into hard-to-reach corners of your home, ultra-fine particles can reach deep into your lungs, affecting your health. This makes it essential to keep our air clean and monitor these tiny particles, just like you would regularly clean your home.
Importance of Measurements and Instrumentation
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This instrument, what it does is it charges all the particles coming in. But depending on the size of the particles, different charges accumulate on particles.
Detailed Explanation
Advanced instruments are used to measure the size and charge of particulate matter. These instruments can differentiate particles based on their size by applying a charge and analyzing how they behave in an electric field. This enables researchers to create detailed profiles of particulate matter in the atmosphere, essential for understanding pollution sources and health impacts.
Examples & Analogies
Imagine a bouncy castle where different balls are placed. The balls are charged and start bouncing around at different rates depending on their size. A specialized observer can determine which balls are bigger and which are smaller based on their bouncing behavior. Similarly, instruments work to distinguish particles by their size and charge to provide valuable data about air quality.
Key Concepts
-
Source Apportionment: It is crucial for identifying and managing pollution sources.
-
Chemical Composition: Provides necessary details for accurate source identification.
-
Measuring PM: Different instruments are designed for various size ranges of particulates.
Examples & Applications
An analysis identifies high levels of ultrafine particles in urban areas, linking them to vehicle emissions.
Using chemical composition data, city planners can develop strategies to reduce pollution from specific sources.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To find the source of pollution in the air, look at composition, and you will care.
Stories
Once in a polluted city, scientists played detective, using chemical composition to unveil the mystery of where the pollution was coming from.
Memory Tools
C.A.P.E. – Composition, Apportionment, Particulate Matter, Emission Sources.
Acronyms
P.E.A.C.E. – Particulate Emissions and Chemical Analysis for Environment.
Flash Cards
Glossary
- Source Apportionment
The process of identifying the origin of pollutants in the atmosphere.
- Chemical Composition
The elements or compounds that make up a substance, providing insight into its source and potential impact.
- Particulate Matter (PM)
Airborne particles that can be solid or liquid and are hazardous to health when inhaled.
- Ultrafine Particulate Matter
Particles that are below 100 nanometers in size, often associated with vehicle emissions.
- Differential Mobility Analyzer
An instrument that sorts particles based on their size and charge.
- Scanning Mobility Particle Sizer (SMPS)
A device used to measure the size distribution of fine particulate matter.
Reference links
Supplementary resources to enhance your learning experience.
