Stack Emission and Plume Formation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Temperature Profile and Vertical Convection
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s start by exploring the temperature profile as a function of height. Can anyone explain how temperature influences air movement?

Is it because warmer air rises while cooler air sinks?

Exactly! This movement is driven by buoyancy due to temperature differences. This process is critical in understanding how pollutants disperse into the atmosphere.

What about when the sun sets? Does that affect the air too?

Great question! As the sun sets, the soil cools rapidly, which can lead to the air above retaining more heat initially. This helps create the temperature inversion effect.

What is temperature inversion?

Temperature inversion occurs when the normal temperature gradient is reversed, with warmer air trapping pollutants close to the ground. This is significant for air quality during cold nights.

So, when does this inversion typically happen?

Inversion can often be observed during nighttime or in cold winter conditions, particularly when conditions are humid. It can lead to fog formation.

To recap, the temperature profile is fundamental in understanding air stability and pollutant transport, which we must consider in environmental quality assessments.
Buoyancy and Air Parcel Dynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's look at buoyancy in more detail. How do buoyancy and temperature interact?

Warm air rises because it’s less dense than cool air.

Correct! When we release a parcel of warmer air, it tends to rise. As it does, it cools and expands. This process is described by the dry adiabatic lapse rate.

What does 'dry adiabatic lapse rate' mean?

'Dry adiabatic lapse rate' is the rate at which rising air cools under adiabatic conditions—specifically, it cools at about 9.8 degrees Celsius per kilometer.

Does this mean all rising air cools at the same rate?

Not exactly. While the dry adiabatic lapse rate is a reference, the actual rate can vary based on humidity and environmental conditions.

What happens if there’s mechanical turbulence?

Mechanical turbulence can push warmer air parcels even higher, enhancing their upward movement. This is critical for understanding pollutant dispersion.

In summary, buoyancy and temperature interactions significantly influence air parcel dynamics, which is essential for predicting plume behavior.
Stable vs. Unstable Conditions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s discuss atmospheric stability—how does it impact pollution concentration in the air?

I think in stable conditions, pollutants stay closer to the surface?

Exactly! In stable conditions, temperature typically doesn't change much with height, causing pollutants to accumulate.

What about unstable conditions?

In unstable conditions, the temperature decreases more rapidly with height, allowing pollutants to disperse more effectively.

So, we want unstable conditions for better air quality?

Correct! Unstable conditions promote dispersion, while stable conditions can trap pollution, leading to higher concentrations.

What do we measure to find out the stability of the atmosphere?

We typically measure the environmental lapse rate and compare it with the dry adiabatic lapse rate to assess atmospheric stability.

In conclusion, understanding stability is vital to predicting how pollutants disperse and remain in the atmosphere.
Mean Mixing Height and Pollutant Dispersion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve into the concept of mean mixing height. Why is it essential for understanding pollutant dispersion?

Is it where the pollutants typically mix in the atmosphere?

Correct! Mean mixing height is the intersection of the dry adiabatic and environmental lapse rates. It indicates where air parcels can effectively mix.

How does this affect our pollution models?

Knowing the mixing height helps in predicting how pollutants spread downwind and assess air quality.

What are factors that can change the mixing height?

Various factors including temperature, humidity, and atmospheric stability can impact mixing height. It varies seasonally and daily.

So, it’s essential for planning pollution control strategies?

Absolutely! Accurate modeling of pollutant dispersion relies heavily on understanding mean mixing height. In summary, it is a critical parameter in air quality management.
Plume Formation Dynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s conclude by examining plume formation. How are plumes created during emissions?

They form when pollutants rise from sources like stacks?

Exactly! The stack provides a source for emissions, and as these pollutants rise, they begin to spread horizontally due to mechanical turbulence and thermal forces.

What affects the shape of the plume?

The plume shape is affected by how much it spreads, which depends on wind direction, atmospheric stability, and thermal dynamics.

So, if the air is stable, the plume stays closer to the ground?

Correct! In stable conditions, plumes are limited and can lead to higher concentrations near the emissions source.

And in unstable conditions, they can spread widely?

Yes, they can disperse more effectively, reducing ground-level concentrations of pollutants. In summary, plume dynamics are influenced by multiple atmospheric conditions and understanding them is key to managing air quality.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides an overview of how temperature variations with height influence air stability and the vertical movement of pollutants, leading to different plume formation scenarios. It examines concepts such as buoyancy, environmental lapse rate, and the impact of mechanical forces on pollutant dispersion.
Detailed
Stack Emission and Plume Formation
This section explores the intricacies of stack emissions and the formation of pollution plumes through temperature profiles in the atmosphere. At the outset, the temperature profile as a function of height is presented, illustrating how the vertical movement of air masses is driven by thermal forces. The discussion centers around the interaction between the temperature of the Earth's surface and the air above it, outlining how these variations lead to environmental lapse rates that can change throughout the day depending on solar radiation.
The concept of temperature inversion is introduced, wherein, during summer days, the soil heats rapidly due to sunlight, creating a positive temperature gradient where air near the surface becomes hotter than the air above. This condition facilitates the upward movement of air parcels, especially when they contain pollutants from sources like generators. The discussion on buoyancy delves into how air parcels with higher temperatures rise due to their lower density compared to cooler surrounding air. Furthermore, it compares stable and unstable atmospheric conditions, evaluating their effects on pollutant diffusion, informing the environmental aspects of air quality.
The concept of mean mixing height is defined as the intersection point of adiabatic and environmental lapse rates, critical for predicting how pollutants disperse in the atmosphere. Lastly, the section analyzes how temperature dynamics and mechanical turbulence influence the horizontal and vertical dispersion of plumes, outlining different scenarios for pollutant concentrations.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Environmental Temperature Profile
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Okay, so, let’s consider two things, first thing to be considered is what is called as the temperature profile as a function of height. So, we are saying that vertical convection happens as a result of thermal forces which means there’s a temperature difference.
Detailed Explanation
This chunk discusses the concept of the temperature profile in the atmosphere, particularly how it varies with height. The temperature profile is a way to understand how temperature changes from the ground up into the atmosphere, which creates vertical convection currents. As the sun heats the earth, the surface temperature increases, creating a temperature gradient where the air close to the ground is warmer than the air above it. This gradient is crucial for understanding how pollutants are transported in the atmosphere.
Examples & Analogies
Imagine a pot of water being heated on a stove. The water at the bottom gets hot first and starts to rise, while cooler water moves down to replace it. This behavior is similar to how air behaves in the atmosphere: warmer air rises, creating convection currents.
Daytime Heating Effects
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
During daytime the radiation heats up the soil or the land faster than it heats the air. So, the radiation directly heats the soil. As a result, the temperature of the soil is very high.
Detailed Explanation
This chunk explains how solar radiation causes the soil to heat up faster than the air above it during the day. As the ground absorbs sunlight, it warms up and raises the temperature of the air directly above it. This creates a positive temperature gradient, which facilitates vertical movement in the atmosphere and impacts how pollutants disperse.
Examples & Analogies
Consider lying on a beach towel on a sunny day. You feel warm against the towel, but the air above you feels cooler. The towel absorbs sunlight and gets hot, warming the air just above it, illustrating the difference in heat absorption rates.
Cooling Process at Night
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The soil then starts cooling, it cools very rapidly and is given up all its heat. Then you see a certain small decrease, the air is still hot but the soil has started cooling.
Detailed Explanation
When the sun sets, the ground releases the heat it absorbed during the day, cooling down rapidly. This results in the air above remaining warmer for a while before ultimately cooling down. This change in temperature profiles affects how air pollutants behave, as cooler air can limit the vertical mixing of pollutants.
Examples & Analogies
Imagine a warm pizza left on a plate. The pizza cools down noticeably faster than the air around it. When you first take it out of the oven, it’s much hotter than the air around it. As it sits there, it cools down, much like the earth does at night.
Temperature Inversion
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Now, this region is called as the temperature inversion. The temperature inversion means generally in the daytime temperature is reducing as a function of height, but here temperature is increasing as height.
Detailed Explanation
A temperature inversion occurs when the typical decrease in temperature with height is reversed. This creates a stable layer in the atmosphere where warmer air sits above cooler air, which can trap pollutants below. Understanding inversions is important for predicting air quality issues, as they can lead to increased concentrations of pollutants.
Examples & Analogies
Think of a warm blanket over a cold body. The warm layer traps heat, preventing it from escaping. Similarly, in a temperature inversion, the warm air traps colder air and pollutants underneath, sometimes leading to smog in cities.
Buoyancy and Air Parcel Movement
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
if I release a parcel here, what happens is, if its temperature is higher, it wants to go up. There are two things at play here. One is buoyancy which is making it go up, but as it goes up if there is no exchange of energy, its volume also expands like this and it cools.
Detailed Explanation
When a parcel of warm air is released, buoyancy (the tendency of less dense, warmer air to rise) causes it to ascend. As it rises, it expands due to lower pressure with altitude and consequently cools down. This cooling process affects its movement and stability within the atmosphere. Understanding these dynamics is crucial for predicting how pollutants disperse after being emitted.
Examples & Analogies
Think of a balloon filled with warm air. When you release it, it rises because the warm air inside is less dense than the cooler air outside. As it rises, the air expands and cools, just like the rising air parcels in our atmosphere.
Atmospheric Stability: Stable, Unstable, and Neutral Situations
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So this is the definition of what people call as the mean mixing height which is the intersection of the adiabatic and environmental lapse rates.
Detailed Explanation
This chunk discusses the concept of atmospheric stability, which can be stable, unstable, or neutral depending on the behavior of air parcels relative to their surrounding environment. In unstable conditions, pollutants can disperse more widely, while stable conditions can lead to pollution accumulation. Identifying the mixing height helps understand how pollutants will spread and mix in the atmosphere.
Examples & Analogies
Imagine a leaf falling from a tree during different weather conditions. On a calm day, it might drift gently to the ground (stable conditions), but on a windy day, it can be blown far away (unstable conditions). Similarly, atmospheric stability affects how far pollutants travel.
Plume Formation and Characteristics
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
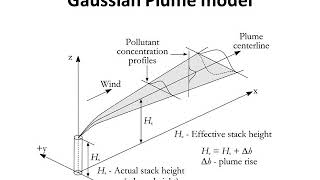
A stack is any source, but it is a very classic stack, you can see that there is an emission occurring here and you can see that it is spreading.
Detailed Explanation
This chunk introduces the concept of a plume, which is the visible trail left by an emission source, like a smokestack. The plume's shape and dispersion depend on both thermal convection and mechanical forces like wind. Understanding how plumes behave is crucial for assessing air quality and the impact of emissions on the environment.
Examples & Analogies
Think of a campfire on a breezy day. The smoke rises and spreads out depending on how hard the wind is blowing. The shape and direction of the smoke plume can tell us a lot about how the fire interacts with the surrounding air, similar to how pollutant emissions behave in the atmosphere.
Key Concepts
-
Stack Emission: The release of pollutants from a stack or chimney into the atmosphere.
-
Temperature Inversion: A phenomenon where cooler air is trapped beneath warmer air, preventing pollutant dispersion.
-
Aerosol Dynamics: The study of how particles behave in the atmosphere, influenced by forces like buoyancy and turbulence.
Examples & Applications
During a summer evening, the land cools, creating a temperature inversion that can lead to fog formation as pollutants are trapped near the surface.
The rising smoke from a chimney illustrates how buoyant air can carry pollutants upward, where they may disperse or react depending on atmospheric conditions.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the air, hot rises high, cool sinks low, watch the sky!
Stories
Imagine a day when the sun warms the soil, causing air balloons (the warm air parcels) to rise, carrying little pollutant passengers. As night falls, the balloons get stuck under a trapped blanket of warm air, holding the pollution close to the ground.
Memory Tools
Use the acronym 'BATS' to remember: Buoyancy, Adiabatic lapse rate, Temperature inversion, Stability for pollutant transport.
Acronyms
PLUME
Pollutant Lift Upwards Mixing Everywhere to remember how plumes form and disperse.
Flash Cards
Glossary
- Temperature Profile
The variation of temperature with height in the atmosphere.
- Environmental Lapse Rate
The rate at which temperature decreases with an increase in altitude in the surrounding atmosphere.
- Buoyancy
The tendency of warmer, less dense air to rise in cooler, denser air.
- Stable Atmosphere
An atmospheric condition where temperature profiles suppress vertical movement of air, trapping pollutants.
- Unstable Atmosphere
An atmospheric condition where temperature facilitates the vertical movement of air, allowing pollutants to disperse.
- Mean Mixing Height
The height at which pollutants are effectively mixed in the atmosphere, influenced by adiabatic and environmental lapse rates.
- Plume
A visible counterpart of the pollutant mass released into the atmosphere, indicating dispersion dynamics.
Reference links
Supplementary resources to enhance your learning experience.
