Environmental Quality: Monitoring and Analysis
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Explaining Vapour Sampling Techniques
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning, everyone! Today we will explore how we can monitor air quality by collecting vapor samples. Can anyone tell me what a Tedlar bag is used for?

Is it used for storing air samples?

Exactly! Tedlar bags are specifically designed to collect and store air samples. Now, why might we prefer using a vapor sample over just grabbing a direct sample?

Because sometimes the concentrations of trace vapors are very low?

Correct! That's why we use adsorbents to concentrate those low levels. The process involves the vapor passing through the adsorbent, where it’s captured. Can anyone remind us why we cap the absorbent tube after sampling?

To prevent the adsorbate from escaping?

Exactly! We want to ensure that our samples are intact for accurate analysis later. Great job!
Extraction Methods: Solvent vs. Thermal Desorption
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss the two primary methods of extracting analytes: solvent extraction and thermal desorption. Can anyone explain what solvent extraction involves?

It’s where we use a liquid to wash out the adsorbed substance?

That's right! We introduce a solvent that competes with the adsorbed analytes. However, what is the disadvantage of using solvents?

Solvents can introduce their own concentrations which might affect the results?

Exactly! On the other hand, with thermal desorption, we just increase the temperature to release what was adsorbed. Does anyone know why temperature is preferred?

Because it doesn’t involve introducing another substance?

Well done! Thermal desorption minimizes sample processing losses and complexities.
Flow Rate and Breakthrough Curves
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In our design for sampling systems, flow rates matter greatly. Can anyone explain what a breakthrough curve represents?

It shows when the adsorbent is saturated, and analytes start to pass through.

Exactly! When the breakthrough occurs, what does that mean for our sampling reliability?

It means we're losing valuable data because the adsorbent can’t capture everything anymore?

Correct! Maintaining proper flow rates helps us avoid going past that threshold. Why do you think precise flow control matters in environmental monitoring?

It ensures accuracy in the concentration levels we measure?

Exactly! It is crucial for maintaining the integrity of our data.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section outlines techniques for sampling ambient air using devices like Tedlar bags and adsorption tubes. It highlights the importance of extraction methods, including solvent and thermal desorption, and addresses factors influencing adsorption efficiency and sampling design.
Detailed
Environmental Quality: Monitoring and Analysis
This section elaborates on the methodologies for sampling ambient air to monitor and analyze environmental quality, focusing on trace vapor organics. It begins with the discussion of canisters and Tedlar bags used for grabbing samples of ambient air. The process involves using adsorbents to capture vapors similarly to PM 10 sampling but employs specific adsorbent materials for effective trapping. Once samples are collected, they require careful handling to prevent loss of analytes during extraction.
Two primary methods for extracting analytes from adsorbents are discussed:
1. Solvent Extraction: This method involves using solvents to recover adsorbates from the adsorbent materials. The procedures discussed draw on principles of partitioning between solid and liquid phases.
2. Thermal Desorption: An alternative approach that minimizes the use of solvents by increasing temperature to release adsorbed compounds, which are then routed directly to analytical instruments.
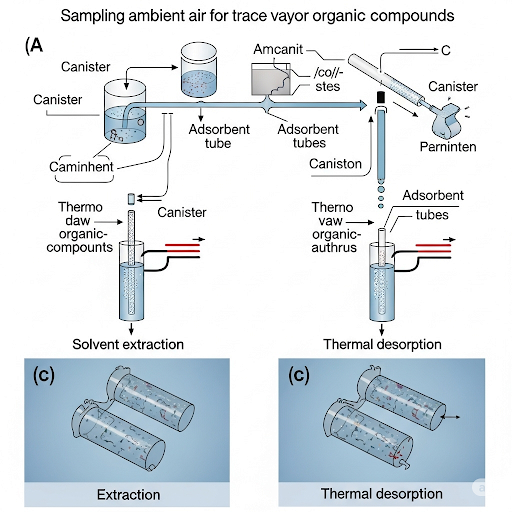
The section emphasizes the significance of flow rates in sampling designs to ensure reliable results, discussing breakthrough curves and adsorbent capacities. Breakthrough represents conditions under which analytes begin to pass through the adsorbent without being captured. Finally, it explores the importance of measuring apparatuses efficiently designed for varying flow rates to ensure effective sampling without loss of analytes.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Sampling Techniques
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For trace vapor organics, you have to accumulate, this is not enough material for you to directly analyze from a grab sample. So, you have to collect enough material and then go to this thing.
Detailed Explanation
In environmental monitoring, especially for trace vapor organics, singular grab samples often don't contain sufficient material to conduct a thorough analysis. Therefore, it is necessary to gather a larger volume of air or material to ensure that the samples are representative and analyzable. This process involves using special equipment to store and collect enough vapor to meet analysis requirements.
Examples & Analogies
Imagine trying to measure the concentration of perfume in a room by just smelling a single spot; you'd need to fill a bottle with air from various locations in the room to get a clear idea of the overall scent.
Using Adsorbents for Sampling
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, the air goes through this adsorbent, there is a PM filter before this. So that only the vapor is allowed to go in. And the analyte A gets trapped on this adsorbent.
Detailed Explanation
When sampling air, the process often involves passing the air through a filter that captures particulate matter (PM) before it reaches the adsorbent. The adsorbent material is specifically designed to hold onto the target compounds (the analyte) from the air, allowing for the analysis of these trapped substances later on.
Examples & Analogies
Think of the adsorbent as a sponge soaking up a liquid. Just like a sponge can only absorb so much water before it gets full, an adsorbent can only hold onto a certain amount of vapor before it becomes saturated.
Extraction Process of Analytes
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Once you have finished this, the absorbent tube is taken out and capped, the ends are closed because you don’t want the adsorbent to leave the system.
Detailed Explanation
After the air sampling process, the adsorbent tube, which has collected the analytes, is carefully sealed to prevent any loss of the trapped substances. This sealing is critical for maintaining the integrity of the sample for further analysis, as any exposure to ambient air could result in loss or alteration of the trapped analytes.
Examples & Analogies
It's like closing a jar after you've collected fresh fruit; leaving it open would result in spoilage or loss of freshness, just as leaving the adsorbent exposed can lead to loss of the valuable samples trapped within.
Desorption and the Role of Temperature
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, this is called as thermal desorption when you do this, which means when you do thermal desorption, you are increasing temperature.
Detailed Explanation
Thermal desorption is a technique used to release the trapped analytes from the adsorbent material. By increasing the temperature, the adsorbate can be vaporized and subsequently analyzed. This makes it possible to recover the compounds that were previously captured without needing a solvent.
Examples & Analogies
Think of how heat is used to cook food. When you heat a pot of water, the increasing temperature causes water vapor to collect and rise, just as increasing temperature in thermal desorption helps release the trapped vapors to be measured.
Challenges with Extraction and Breakthrough
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If you extract this and I get some concentration corresponding to this tube, whatever there in this tube, I also know the volume (Q×Δt).
Detailed Explanation
When analyzing the concentration of substances in a sampled volume, it's crucial to understand whether the sample accurately represents what was in the environment. Factors such as adsorbent saturation and flow rate can affect the effectiveness of the sampling, necessitating a well-designed procedure that considers potential losses.
Examples & Analogies
It's similar to filling a glass with water from a fountain. If the flow is too fast, the glass might overflow before it fills completely, meaning you won't get an accurate measure of how much water the fountain produces over time.
Importance of Monitoring Breakthrough
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When breakthrough happens, it means that the sampling is now ineffective because it has gone through the column.
Detailed Explanation
Breakthrough refers to the point at which the adsorbent has become saturated, and additional analytes can no longer be captured effectively. Understanding this concept is vital in sampling design to ensure that monitoring remains accurate and that results are reflective of ambient conditions.
Examples & Analogies
Imagine trying to use a sponge to clean up a spill; once the sponge is full, it can't absorb any more liquid, and everything afterward will just flow off onto the floor. Similarly, if breakthrough occurs, all the analytes will bypass the adsorbent, leading to invalid sample results.
Key Concepts
-
Vapor Sample Collection: Using specialized equipment like Tedlar bags to gather air samples.
-
Adsorbent Materials: The use of materials that can capture vapor samples for analysis.
-
Extraction Techniques: Methods like solvent extraction and thermal desorption are employed to retrieve analytes.
-
Flow Rate Importance: Adjusting flow rates is crucial to avoid breakthrough and ensure accuracy in sampling.
Examples & Applications
Using a Tedlar bag to collect vapor samples from a landfill site to determine volatile organic compounds (VOCs).
Employing thermal desorption techniques to analyze air samples collected from urban environments.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To capture air from spaces rare, Tedlar bags are always there.
Stories
Imagine if you need to capture the scent of flowers at a garden. You use a Tedlar bag, similar to a vacuum, to hold the fragrance intact for later aroma testing.
Memory Tools
USE Adsorbents: Understand, Sample, Extract - to remember the steps of vapor sampling.
Acronyms
STEADY
Sampling
Trapping
Extracting
Analyzing
Desorbing
Yielding results - to represent the process.
Flash Cards
Glossary
- Tedlar bag
A specialized bag used to collect air samples without contamination.
- Adsorption
The process by which atoms or molecules from a gas adhere to a solid surface.
- Adsorbent
A material used to capture and hold other substances on its surface.
- Solvent extraction
A method of separating a substance from one phase to another using a solvent.
- Thermal desorption
A technique that raises the temperature of a substance to release adsorbed materials for analysis.
- Breakthrough curve
A graph that plots the concentration of an analyte exiting an adsorbent over time, indicating when saturation occurs.
- Flow rate
The volume of fluid that passes through a given surface per unit time; crucial for effective sampling.
Reference links
Supplementary resources to enhance your learning experience.
