Sampling/Adsorption and Extraction/Desorption
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Sampling and Adsorption
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore how sampling and adsorption work in monitoring trace vapor organics. Can anyone explain why adsorbents are necessary for this process?

Adsorbents trap the analytes from the air because the concentration is usually very low.

Right! So, how do we actually collect the samples?

Great question! We typically use canisters or Tedlar bags with pumps to draw in enough air. This method ensures we have a significant volume for analysis.

Why do we have to use different adsorbents?

Excellent! Different adsorbents have varying affinities for specific compounds, which helps optimize the trapping and future analysis. Remember the concept of 'affinity'—it relates to how 'sticky' the adsorbent is for certain vapors.

So higher affinity means better trapping capability?

Exactly! Higher slopes in the adsorption graphs indicate better adsorption capacity.
Desorption Techniques
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's move on to desorption methods. Can anyone explain the need for desorption?

It’s to remove the trapped analytes so we can analyze them!

Do we always need solvents for this?

Not always! While solvents can help, we can also employ thermal desorption, which uses heat to release the analytes without additional solvents.

Doesn't that require a lot of energy?

Good observation! Thermal desorption can be energy-intensive, especially if vacuum conditions are involved, but it avoids complications with solvent handling.

How do we ensure the analytes don't escape before we can analyze them?

That's a key point. It's essential to control conditions precisely so we can collect all vapor that is released during desorption.
Importance of Breakthrough Curves
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we are discussing breakthrough curves. Who can explain what breakthrough means in our context?

It’s when the concentration of analytes in the effluent matches that of the influent!

And that means the adsorbent is fully saturated?

Exactly! Breakthrough tells us when we can no longer effectively capture the analytes. Monitoring this can help avoid inaccurate results.

So, if we design a sampling system, we need to consider flow rates to prevent exceeding adsorbent capacity, right?

Spot on! The effectiveness of our sampling depends not just on the adsorbent but the flow rates as well.

What happens if the breakthrough occurs?

In that case, we must stop sampling immediately because the accuracy of our measurement will be compromised.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides an overview of the techniques involved in sampling ambient air for trace vapor organics using adsorbents, the necessity of accumulating sufficient material for analysis, and the subsequent methods for extraction and desorption. It emphasizes the importance of selecting appropriate adsorbent materials and the conditions required for effective sampling and desorption.
Detailed
Sampling/Adsorption and Extraction/Desorption
This section explores the critical processes of sampling and adsorption in the context of trace vapor organics in environmental monitoring. The initial stage involves capturing ambient air into either a canister or a Tedlar bag, with the aid of a pump, to gather enough vapor for analysis. Given the low concentrations of trace organics, an adsorbent material is vital to trap the analytes effectively while filtering particulate matter using filter paper.
Adsorption dynamics are discussed using a graph illustrating the relationship between concentration in the air and adsorbate uptake on the adsorbent. The slope of this graph indicates the adsorption capacity; higher slopes correlate with better adsorption performance.
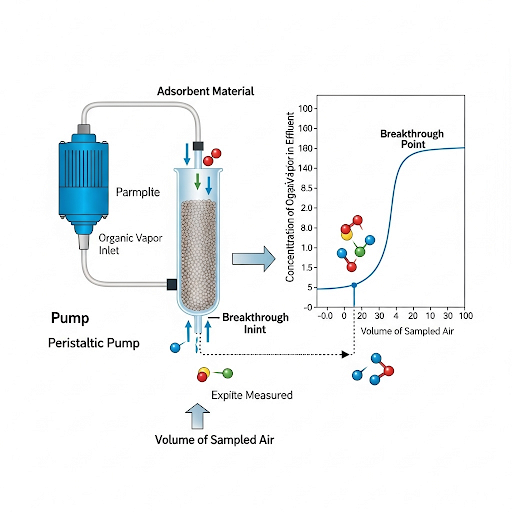
Following sampling, the section addresses desorption methods, where the trapped analytes must be extracted from the adsorbent. This may involve using solvents or altering temperature and pressure conditions, with a preference for thermal desorption which simplifies the process by avoiding additional solvents. The importance of managing the sampling and adsorbent saturation is highlighted, with breakthrough curves illustrating how adsorbents perform over time, ensuring that accurate concentrations are understood throughout sampling procedures.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Sampling and Adsorption
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For trace vapor organics, you have to accumulate, this is not enough material for you to directly analyze from a grab sample. So, you have to collect enough material and then go to this thing. So, what is generally done is the vapor sample is drawn just like the way we do for PM 10 sampling, we collect on a filter paper, we do have a filter and this filter is an adsorbent. And different adsorbent materials are available that you can use to trap. So, the air goes through this adsorbent, there is a PM filter before this. So that only the vapor is allowed to go in. And the analyte A gets trapped on this adsorbent.
Detailed Explanation
To analyze trace vapor organics in the air, we cannot simply grab a sample; we need to collect a sufficient volume for accurate analysis. This is done using a method similar to PM 10 sampling. In this process, a vapor sample is drawn through a filter paper that contains an adsorbent material. This filter is crucial because it allows only vapor to pass through, trapping the analyte A on the adsorbent for later analysis.
Examples & Analogies
Think of this process like using a coffee filter. When you brew coffee, the filter allows only the liquid coffee to pass through while trapping coffee grounds. Similarly, in vapor sampling, the filter allows vapor to enter while capturing specific analytes.
Desorption Process
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Once you have finished this, the adsorbent tube is taken out and capped, the ends are closed because you don’t want the adsorbent to leave the system you want it to stay there so that you at least want to isolate it, and then the analyte is extracted. Typically, when you want to extract it, you use the same kind of procedures we have been using for extraction for solids and other things water as solvent.
Detailed Explanation
After trapping the analyte on the adsorbent tube, it is carefully capped to prevent any loss of material. This step is crucial for maintaining the integrity of the sample. To extract the trapped analyte effectively, we often use a solvent, similar to how we extract compounds from solid materials using liquids. The liquid solvent helps pull the analyte off the adsorbent for analysis.
Examples & Analogies
Imagine using a sponge to soak up a spill. When you want to release the liquid from the sponge, you might squeeze it out. In the same way, we use a solvent to 'squeeze' the analyte out of the adsorbent material.
Adsorbent Selection and Performance
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Here we are talking about the adsorption. We want to select a material for the adsorbent from air which has something as shown in the graph, the slope is very high. The slope of this is what we call as K is the ratio. So, higher the slope, which means the higher adsorption capacity, so, it will take a lot more, equilibrium is towards the solid.
Detailed Explanation
When selecting an adsorbent material for sampling, it’s important to consider its adsorption capacity. This capacity is represented by the slope of a graphical relationship called the adsorption isotherm. A steeper slope (higher K value) indicates that the adsorbent can capture more analyte from the air, meaning it is more effective. The process tends to favor the transfer of vapor from the gas phase to the solid phase, which is the primary goal during sampling.
Examples & Analogies
Think of trying to absorb water with different types of cloths. A thicker, denser towel will absorb more water (higher adsorption capacity) than a thin handkerchief. Similarly, our choice of adsorbent influences how much vapor we can capture for analysis.
Desorption Conditions
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When you want to extract it, you want to have the extraction is something like this where you want to get everything off the solid. So, which means that now you have to bring it in contact with something else and you can’t do extraction in air obviously.
Detailed Explanation
Desorbing the analyte from the adsorbent requires specific conditions that cannot simply be achieved in the air. This process involves changing the environment around the adsorbent, such as introducing a solvent, to ensure that the analyte is effectively released. Proper desorption is crucial for accurate analysis, as the analyte must be fully removed from the solid phase to be measured accurately.
Examples & Analogies
Think of a stubborn stain on fabric. To fully remove the stain, you often need to apply a specific cleaning solution, rather than just using water. In the same way, releasing an analyte from an adsorbent requires the right conditions to ensure complete extraction.
Methods of Desorption: Solvent vs. Thermal
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
One option is to use a solvent. What you are doing in extraction is this so, we go back to our partitioning this thing. One way is to use a solvent...But the second way, if you do not want to bring another solvent into question, because once you bring a solvent, you have to look at solvent concentration and evaporation of solvent and all these issues are there sample processing losses are more.
Detailed Explanation
There are two primary methods for desorbing an analyte from an adsorbent: using a solvent or applying thermal energy (heat). Using a solvent helps to effectively extract the analyte but introduces complexities like solvent evaporation and concentration issues. Alternatively, thermal desorption uses heat to release the analyte without introducing additional solvents, which simplifies the process.
Examples & Analogies
Consider how you can clean a greasy pan. You can either soak it in soapy water (solvent method) or heat it until the grease loosens (thermal method). Both methods can be effective, but the soaking method may involve more steps to manage the soap and water.
Key Concepts
-
Sampling: The method of collecting air samples using canisters or Tedlar bags.
-
Adsorption: The process where analytes adhere to an adsorbent material used during sampling.
-
Desorption: Removing analytes from an adsorbent for analysis.
-
Breakthrough: The saturation point where collected analytes in the adsorbent match the concentration in the air.
Examples & Applications
Using a Tedlar bag for air sample collection allows for effective containment of trace organics.
When using a charcoal adsorbent, the adsorption capacity can vary based on the composition of the air.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To trap the air, adsorb with care; breakthrough means we might despair.
Stories
Imagine a sponge soaking up water; once saturated, it can hold no more without spilling.
Acronyms
Remember A-D-B
Adsorb
Desorb
Breakthrough help with sampling safety!
ADAPT - Adsorbents, Desorption, Adsorption process, Temperature effect.
Flash Cards
Glossary
- Adsorption
The process where molecules adhere to the surface of a solid or liquid.
- Desorption
The process of removing adsorbed molecules from a solid or liquid.
- Breakthrough
The point at which the concentration of analytes in the effluent equals that in the influent, indicating adsorbent saturation.
- Tedlar Bag
A type of bag used for collecting and storing air samples, made from a specialized plastic.
Reference links
Supplementary resources to enhance your learning experience.
