The Mole Concept and Avogadro’s Constant
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the Mole Concept
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning, class! Today, we are going to explore the fundamental concept of the mole. Can anyone tell me why the mole is important in chemistry?

Is it because it helps us measure small particles that we can't see directly?

Exactly! The mole allows us to relate the mass of a substance to the number of atoms or molecules it contains. One mole is defined as 6.02214076 × 10²³ entities of that substance—this is known as Avogadro's constant.

How did they come up with that number?

Great question! This definition originated from the mass of carbon-12. By setting 12 grams of carbon-12 equal to exactly one mole, it provides a bridge between the macroscopic world and the microscopic scale of atoms.

So, if we have a mole of any element, we can calculate its mass using the periodic table, right?

Yes! The molar mass in grams per mole is numerically equal to the relative atomic mass on the periodic table. Let's remember that: 'Molar mass is mass per mole, just read the table to know!'

Before we move on, can anyone summarize why the mole is critical in chemistry?

The mole helps us convert between mass, volume, and the number of particles, which is essential for predicting how reactions will occur.

Perfect summary! Remember, understanding the mole concept is essential as we delve deeper into stoichiometry.
Molar Mass and Conversions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss how to use molar mass in conversions. Who can tell me how we convert grams to moles?

We divide the mass of the substance by its molar mass!

Correct! The formula is: Number of moles = mass (g) / molar mass (g/mol). For example, how many moles are in 25 grams of sodium chloride (NaCl)?

First, I find the molar mass of NaCl, which is about 58.44 g/mol, right?

Exactly! Now, what’s the next step?

We can plug in: Number of moles = 25 g / 58.44 g/mol, which gives us about 0.428 moles.

Well done! Now let’s look at converting moles back to mass. If we have 2 moles of water (H₂O), how do we find its mass?

We multiply the number of moles by its molar mass. The molar mass of water is about 18.02 g/mol.

Correct! So what's the final calculation?

It would be 2 moles * 18.02 g/mol, which equals 36.04 grams!

Great! Remember, these conversions will be key in stoichiometric calculations. Let's summarize: 'To find moles, divide the mass by molar mass; to find mass, multiply by molar mass!'
Practical Examples of Mole Conversions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s work through some practice examples together! First example: How many oxygen atoms are in 5 grams of O₂?

First, we find the molar mass of O₂, which is 32.00 g/mol. Then we calculate the moles: moles = 5 g / 32 g/mol.

Exactly! What do you get?

That gives me 0.15625 moles of O₂.

Correct! Now, how do we find the number of O₂ molecules?

We multiply by Avogadro's number: 0.15625 mol × 6.022 × 10²³ molecules/mol.

Right! What’s the result?

That gives about 9.41 × 10²² molecules of O₂.

Well done! Now consider: how many oxygen atoms are in that amount of O₂?

Since each O₂ molecule has 2 oxygen atoms, we multiply 9.41 × 10²² by 2, giving us 1.88 × 10²³ oxygen atoms!

Excellent! This example illustrates how to go from grams to number of atoms. Remember your steps: convert grams to moles, then moles to molecules, then to atoms.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the importance of the mole in bridging the macroscopic and microscopic worlds in chemistry. It defines the mole as containing Avogadro's number of entities and details how to convert between mass, moles, and the number of entities. Additionally, it introduces molar mass and mass-mole conversions as foundational concepts in stoichiometry.
Detailed
The Mole Concept and Avogadro’s Constant
The mole is the fundamental unit of measurement in chemistry, enabling scientists to convert between the mass of a substance and the number of atoms or molecules it contains. By definition, one mole of any substance contains exactly 6.02214076 × 10²³ elementary entities (Avogadro's constant). The historical basis of this definition ties back to carbon-12, where 12 grams equates to this exact number of atoms.
Key Areas Covered:
- The Definition of the Mole: A mole directly relates macroscopic measurements (grams) to microscopic quantities (atoms, molecules) through a fixed number of entities.
- Molar Mass: This is the mass of one mole of a substance, expressed as grams per mole (g/mol). Molar mass allows for conversions between mass and number of moles, with formulas defining:
- Number of moles = mass (g) / molar mass (g/mol)
- Conversion Examples: Practical exercises demonstrate how to convert mass to moles, moles to number of particles, and vice versa, emphasizing the significance of these calculations in chemical reactions and stoichiometry.
Understanding the mole concept is crucial for further studies in chemistry as it facilitates quantitative calculations essential for predicting reaction outcomes and yields.
Youtube Videos
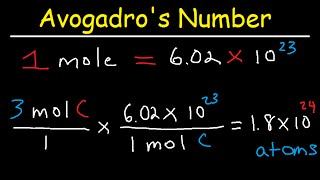

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Why the Mole? From Atoms to Grams
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Macroscopic vs. microscopic quantities
- A single sodium (Na) atom has a mass of approximately 3.82 × 10⁻²³ g. Measuring individual atoms directly in the laboratory is impossible.
- Chemists define the mole to bridge these scales: one mole of any substance contains a fixed, very large number of “entities” (atoms, molecules, ions, electrons, etc.), allowing us to weigh out amounts in grams.
- Definition of the mole
- By definition, 1 mol of a substance is the amount of that substance that contains exactly 6.022 140 76 × 10²³ elementary entities. This number is called Avogadro’s constant (NA).
- Avogadro’s constant, NA = 6.022 140 76 × 10²³ entities per mole
- Historical basis
- The mole was originally defined so that exactly 12 g of pure carbon-12 (¹²C) contains 6.022 140 76 × 10²³ carbon atoms. This definition ties the atomic mass unit (1 u ≈ 1.660 539 × 10⁻²⁷ kg) to a macroscopic mass.
Detailed Explanation
This chunk introduces the concept of the mole and its significance in bridging the gap between the microscopic world of atoms and the macroscopic world we can measure. A single sodium atom has a tiny mass (around 3.82 × 10⁻²³ grams), making it impractical to measure individual atoms in a lab setting. To facilitate scientific calculations, chemists use the mole as a unit of measurement, where one mole corresponds to Avogadro's constant (approximately 6.022 × 10²³ entities). This allows chemists to weigh out substances in grams instead of dealing with individual particles, which is unattainable. It also ties the mole to the atomic mass of carbon-12, establishing a practical link between the microscopic and macroscopic worlds.
Examples & Analogies
Think of the mole like a dozen eggs. Just as a dozen means 12 eggs, one mole refers to 6.022 × 10²³ entities, whether they're atoms or molecules. Imagine trying to buy a single egg at the grocery store; it would be cumbersome. Instead, you buy a dozen. Similarly, in chemistry, the mole allows scientists to work with a manageable number of particles that we can weigh and measure in a lab.
Molar Mass and Mass ⇄ Mole Conversions
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Atomic mass and molar mass
- On the periodic table, the number under each element symbol (relative atomic mass) tells us how many atomic mass units a single atom has (for naturally occurring isotopic mixtures).
- By convention, a relative atomic mass in atomic mass units (u) is numerically identical to the molar mass in grams per mole (g/mol).
- Example: Carbon (C) has a relative atomic mass of 12.011 u; thus, its molar mass is 12.011 g/mol.
- Chlorine (Cl) appears as 35.45 u because it is a mixture of ³⁵Cl and ³⁷Cl isotopes. Therefore, 1 mol of natural chlorine atoms has a mass of 35.45 g.
- Molecular and formula mass
- For molecules, add the atomic masses of each constituent atom to find the molecular mass (in u). For ionic compounds or empirical formulas, the sum is called the formula mass (in u).
- Example: Water, H₂O.
- Mass of 2 H = 2 × 1.008 u = 2.016 u
- Mass of 1 O = 16.00 u
- Molecular mass of H₂O = 2.016 u + 16.00 u = 18.016 u
- Therefore, the molar mass of H₂O is 18.016 g/mol.
Detailed Explanation
This chunk explains how to convert between grams and moles using molar mass. The atomic mass listed on the periodic table represents both the mass of a single atom in atomic mass units (u) and the molar mass in grams per mole (g/mol). For instance, carbon has a relative atomic mass of 12.011 u, which means one mole of carbon weighs 12.011 grams. Similarly, the molecular mass of compounds can be calculated by summing the masses of the individual atoms in a molecule. If you take water (H₂O) as an example, its molecular mass is 18.016 u, which corresponds to a molar mass of 18.016 g/mol.
Examples & Analogies
To understand molar mass, let's use a grocery bag as an analogy. If you think of atomic mass like the weight of an individual apple measured in grams, the amount of apple in your grocery bag (one dozen apples) represents the total weight that we call the molar mass. Just as you can measure apples in kilograms, chemists use molar mass to translate the microscopic world of atoms into useful quantities we can work with in the lab.
General Mass–Mole Relationships
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Mass to moles:
- number of moles = mass (g) / molar mass (g/mol)
- Moles to mass:
- mass (g) = number of moles (mol) × molar mass (g/mol)
- Moles to number of entities:
- number of entities = number of moles (mol) × NA
- Number of entities to moles:
- number of moles (mol) = number of entities / NA
- Practice Example 1: Converting mass of substance to number of atoms
- Problem: How many oxygen atoms are present in 5.00 g of O₂ gas?
- Compute the molar mass of O₂:
- Mass of one O atom = 16.00 u = 16.00 g/mol. Therefore, molar mass of O₂ = 2 × 16.00 g/mol = 32.00 g/mol.
- Convert mass of O₂ to moles:
- Number of moles of O₂ = 5.00 g ÷ 32.00 g/mol = 0.15625 mol.
- Convert moles of O₂ to number of O₂ molecules:
- Number of O₂ molecules = 0.15625 mol × 6.022 × 10²³ molecules/mol = 9.41 × 10²² molecules.
- Each O₂ molecule contains 2 oxygen atoms. Therefore,
- Number of oxygen atoms = 9.41 × 10²² molecules × 2 atoms/molecule = 1.882 × 10²³ atoms.
- Answer: 1.88 × 10²³ oxygen atoms.
Detailed Explanation
In this chunk, we focus on key formulas for converting between mass, moles, and entities, emphasizing relationships that chemists frequently use. To determine the number of moles from mass, divide the mass of the substance by its molar mass. Conversely, to find mass from moles, multiply the number of moles by the molar mass. The chunk also illustrates how to convert between moles and the number of entities using Avogadro's number (NA). Finally, it provides a practice example showing how to calculate the number of oxygen atoms from a given mass of oxygen gas, demonstrating the practical application of these relationships.
Examples & Analogies
Think of moles like a box of chocolates. If each box contains 12 chocolates, and you want to calculate the number of chocolates you have when you know how many boxes (moles) you have, you simply multiply the number of boxes by 12 to find the total chocolates (entities). Conversely, if you have a certain weight of chocolates (mass), you can divide by the weight of chocolates in each box (molar mass) to find out how many boxes you have, and thus how many chocolates in total.
Key Concepts
-
The mole is a fundamental counting unit in chemistry, allowing conversions among mass, volume, and particle counts.
-
Avogadro's constant defines how many entities are present in one mole of any substance (approximately 6.022 × 10²³).
-
The mass of one mole of a substance is referred to as its molar mass and is used to convert between mass and moles.
Examples & Applications
To find the number of moles in 25 grams of calcium carbonate (CaCO₃), divide the mass (25 g) by its molar mass (100.09 g/mol), resulting in approximately 0.2498 moles.
To calculate the number of entities in 0.5 moles of carbon, multiply the moles (0.5 moles) by Avogadro's constant (6.022 × 10²³ entities/mole), resulting in approximately 3.01 × 10²³ atoms.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In one mole, you see, Avogadro's magic key, to count atoms with such ease, 6.022, that’s the tease!
Stories
Imagine a chemist named Ava who loved to count. One day she found a magical box that contained 6.022 × 10²³ tiny particles. She called it Avogadro’s box and used it to help her friends count their elements too.
Memory Tools
To remember mole calculations: 'Mary's Happy Math Monster' - M for Moles, H for How many, M for Mass.
Acronyms
M.A.C. - Molarity, Avogadro's, Conversion. A way to remember the three main principles we're using!
Flash Cards
Glossary
- Avogadro's Constant
The number of entities in one mole of a substance, approximately 6.022 × 10²³.
- Mole
The amount of substance that contains 6.022 × 10²³ entities.
- Molar Mass
The mass of one mole of a substance, expressed in grams per mole (g/mol).
- Conversion
The process of changing from one unit to another, such as mass to moles.
- MassMole Relationship
The relationship that allows the conversion between the mass of a substance and the amount in moles.
Reference links
Supplementary resources to enhance your learning experience.
