Group 17: Halogens
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Halogens
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will focus on Group 17, known as the Halogens. Who can tell me what the electronic configuration of the Halogens is?

They have an ns² np⁵ configuration, right?

Exactly! This configuration means they are one electron short of a full octet, making them highly reactive. Can anyone name the Halogens?

Fluorine, Chlorine, Bromine, Iodine, and Astatine!

Great job! Now, let’s remember that the reactivity of these elements comes from their desire to gain one more electron. A mnemonic to remember them is 'Fabulous Clowns Bring Incredible Adventures'—F, Cl, Br, I, At. Why do you think they react more vigorously with metals?

Because they want to gain an electron to achieve a stable configuration?

Correct! They can form ionic halides by gaining that electron. Let's summarize key points: Halogens are highly reactive nonmetals with ns² np⁵ configurations, found as various states at room temperature.
Physical Properties of Halogens
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss the physical properties of the Halogens. At room temperature, they exist in different states. Can anyone tell me their states?

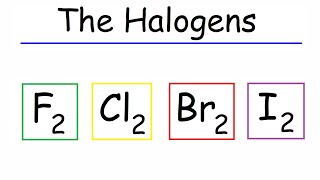
F₂ and Cl₂ are gases, Br₂ is a liquid, and I₂ and At₂ are solids!

Perfect! And the colors vary too. Fluorine is pale yellow, Chlorine is greenish-yellow, Bromine is reddish-brown, and Iodine is grey-black. Why do you think the colors intensify as you move down the group?

Maybe because of their molecular structures and changes in density?

Yes, as the atoms get larger, the van der Waals forces strengthen, affecting color and state. Let’s summarize: Halogens are gases, liquids, or solids, with depth of color increasing down the group—from F to I. Remember the saying 'Gases Are Light' to recall their states.
Chemical Reactivity of Halogens
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we will look at the chemical reactivity of Halogens. They are known for reacting with metals. What do they commonly form?

Ionic halides, like NaCl.

Correct! They also react with hydrogen to form hydrogen halides such as HCl. Can anyone point out how the strength of these oxidizers changes down the group?

Fluorine is the strongest oxidizing agent, and it decreases to iodine.

Exactly! Fluorine can even oxidize water. Now, regarding electron affinity, what's the trend down the group?

It generally decreases because of increased electron-electron repulsion, especially in smaller atoms.

Right! This is why the trend varies, especially with Fluorine being less exothermic in electron affinity than Chlorine. Let’s summarize: Halogens react with many elements to form compounds, and their strength as oxidizers diminishes from top to bottom in the group.
Trends and Properties in Halogens
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss trends in boiling and melting points among the Halogens. What do you notice about their physical states and points?

They increase as you go down the group!

Exactly! This is due to the strengthening van der Waals forces. How about the acidity of hydrogen halides?

HF is the weakest acid, and the strength increases in the order HF < HCl < HBr < HI!

Correct! The bond strength decreases from HF to HI, increasing acidity. To remember it, think 'Happy Cats Bring Happiness'. Let’s summarize our key points: Boiling points and acidity increase down the group, and there is a trend in their compounds forming stronger acids as we go from F to I.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The Halogens are characterized by their ns² np⁵ electron configurations, making them one electron short of a full octet. They exhibit varied physical states at room temperature, reactivity with metals to form ionic halides, and decreasing oxidizing power down the group as well as trends in electron affinity and boiling points.
Detailed
Group 17: Halogens
Group 17 of the Periodic Table encompasses the Halogens, including Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At). This group displays a common electronic configuration of ns² np⁵, indicating that they are highly electronegative and form strong oxidizing agents.
Physical Properties
- At room temperature, the Halogens exist in various states: F₂ and Cl₂ are gaseous, Br₂ is liquid, and I₂ and At₂ are solid. The colors of these elements deepen down the group; fluoro-gas emits a pale yellow, while iodine appears grey-black with purple vapor.
Chemical Reactivity
- The Halogens react vigorously with metals to create ionic halides (like NaCl) and with hydrogen to produce hydrogen halides (e.g., HCl). Their oxidizing ability decreases from fluorine (the strongest) to iodine (the weakest), correlating inversely with standard reduction potentials.
Trends
- Electron affinity tends to decrease down the group due to increased electron-electron repulsion, particularly in fluorine where the electron cloud is compact, and the added electron experiences greater repulsion compared to chlorine. The boiling and melting points also increase downward as van der Waals forces strengthen. Furthermore, the acidity of hydrogen halides increases in the order HF < HCl < HBr < HI as bond strength weakens.
In summary, Group 17 represents a fascinating aspect of the periodic table where nonmetals display a variety of states, reactivity, and trends that are crucial for understanding their behaviors in chemical reactions.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Electronic Configuration
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● ns² np⁵ valence shell (one electron short of a filled octet).
● Highly electronegative and electron-affinic → strong oxidizing agents.
Detailed Explanation
Halogens belong to Group 17 of the periodic table and have an electron configuration of ns² np⁵. This means they have 7 electrons in their outer shell, just one short of a complete octet which makes them very reactive. Their high electronegativity means they have a strong tendency to attract electrons from other elements, making them effective oxidizing agents. Because they are so close to having a full valence shell, they readily gain an electron during chemical reactions.
Examples & Analogies
You can think of halogens like a team of workers who are just one person short of being fully staffed. They are eager to recruit that last member to complete their team, similar to how halogens want to gain one more electron to achieve stability. This is why they react readily with metals and other elements.
Physical Properties
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Exist as diatomic molecules at room temperature:
○ F₂ and Cl₂ are gases; Br₂ is a liquid; I₂ and At₂ are solids (sublime to gas upon heating).
● Colours deepen down the group: F₂ (pale yellow), Cl₂ (greenish-yellow), Br₂ (reddish-brown liquid, reddish vapour), I₂ (grey-black solid with purple vapour).
Detailed Explanation
The halogens are unique in that they primarily exist as diatomic molecules (e.g., F₂, Cl₂) at room temperature. Fluorine (F₂) and chlorine (Cl₂) are gases, bromine (Br₂) is a liquid, and iodine (I₂) is a solid that sublimates to form a gas with a purple vapor when heated. The colour of these gases becomes darker as you go down the group, from pale yellow in fluorine to deep purple in iodine. This trend can be attributed to the increasing intermolecular forces (Van der Waals forces) as the size of the halogen increases.
Examples & Analogies
Imagine looking at different shades of rainbow colors. Just like how certain colors deepen as you go from light pastels to rich dark hues, halogens display deeper colors as you move from fluorine to iodine. When you see vividly colored vapors during experiments, you can connect them back to their positions in the group.
Chemical Reactivity
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● React vigorously with metals to form ionic halides (e.g., 2Na + Cl₂ → 2NaCl).
● With hydrogen, form hydrogen halides (e.g., H₂ + Cl₂ → 2HCl).
● With organic compounds, undergo substitution or addition (in advanced organic chemistry).
● Oxidizing power decreases down the group:
○ F₂ is the strongest oxidizing agent (can even oxidize water to O₂), then Cl₂, Br₂, I₂.
○ Standard reduction potentials: F₂/F⁻ (+2.87 V), Cl₂/Cl⁻ (+1.36 V), Br₂/Br⁻ (+1.07 V), I₂/I⁻ (+0.54 V).
Detailed Explanation
Halogens are known for their vigorous reactions. They react with metals to form ionic compounds called halides, such as table salt (NaCl), through a transfer of electrons. They also react with hydrogen to form hydrogen halides, which are important in various chemical processes, including the production of strong acids such as hydrochloric acid (HCl). The oxidizing power of halogens decreases from fluorine to iodine; fluorine is the most powerful oxidizer, which means it can prompt other substances to lose electrons while it gains them. This is important in oxidation-reduction reactions.
Examples & Analogies
Think of halogens like very aggressive recruiters trying to hire the best talent; they react with metals (the candidates) to form robust compounds (the end products). Fluorine is the most energetic recruiter, aggressively seeking out potential candidates from a big talent pool and grabbing them before anyone else can.
Trends within Group 17
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Electron affinity generally decreases down the group (F is less exothermic than Cl due to strong electron–electron repulsion in small 2p orbitals).
● Boiling and melting points increase down the group (increasing van der Waals forces).
● Acid strength of hydrogen halides: HF (weak acid) < HCl < HBr < HI (stronger acids as bond strength decreases).
Detailed Explanation
As you move down the group in the halogens, some trends are observed. The electron affinity, or the energy released when an atom gains an electron, decreases. This is particularly notable as fluorine, the smallest halogen, experiences repulsion in its compact orbitals when an electron is added. Moreover, the boiling and melting points of the halogens increase down the group due to the greater size and mass leading to stronger van der Waals forces between the molecules. The strength of acids formed from hydrogen halides also increases, demonstrating stronger acidic nature from HF to HI, primarily due to the stability of the bonds.
Examples & Analogies
Imagine a growing series of balloons filled with air. The larger the balloon (deeper down the group), the more air it can hold without bursting. Just as larger balloons experience greater pressure keeping them intact, halogen molecules experience increasing van der Waals forces that require more energy to separate them as you move down the group.
Special Cases
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Fluorine: Most reactive nonmetal; extremely electronegative (χ = 3.98). Reacts with almost all elements (even noble gases under extreme conditions).
● Chlorine: Widely used as a disinfectant (bleach, water purification).
● Astatine: Rare, radioactive; chemistry not well explored.
Detailed Explanation
In the halogen group, certain elements stand out. Fluorine is known as the most reactive nonmetal and incredibly electronegative, meaning it has a strong affinity for electrons and will react with almost all elements, including some noble gases in rare circumstances. Chlorine is well-recognized for its disinfectant properties and is commonly used in bleach and water purification processes. Astatine, on the other hand, is a relatively rare and radioactive element, and thus not much of its chemistry is well understood.
Examples & Analogies
Think of fluorine as a hyperactive child in a room full of toys—wanting to play with everything and extremely enthusiastic about grabbing every new toy (electron). Chlorine, like a diligent cleaner, ensures everything is sanitized and safe, while astatine, being a rare find, is like a hidden treasure that not many have had the chance to explore.
Key Concepts
-
Halogens: Elements with ns² np⁵ configurations, highly reactive nonmetals.
-
Reactivity: They form ionic compounds with metals and hydrogen halides.
-
Electron Affinity: Decreases down the group due to increased electron-electron repulsion.
-
Boiling and Melting Points: Increase down the group as Van der Waals forces strengthen.
-
Acidity: Strength of hydrogen halides increases down the group as bond strength decreases.
Examples & Applications
Fluorine reacts with sodium to form sodium fluoride (NaF), a common ionic halide.
Hydrogen chloride (HCl) is formed when chlorine reacts with hydrogen, demonstrating the trend in hydrogen halides.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Fluorine, Chlorine, make things bright; Bromine flows, Iodine's in sight!
Stories
Once upon a time in the Land of Halogens, Fluorine was the mightiest warrior, defeating metals with ease. Chlorine, a bit less fierce, still helped make water safe. Bromine liked to flow on warm days, while Iodine preferred to stay solid but would turn into a vapor of purple when the heat was right.
Memory Tools
Remember the 'Fabulous Clowns Bring Incredible Adventures' for F, Cl, Br, I, At.
Acronyms
Use 'H-B-H-F' to remember hydrogen halides' acidity
HF < HCl < HBr < HI.
Flash Cards
Glossary
- Halogens
Group 17 elements, characterized by their ns² np⁵ electron configuration and high reactivity.
- Electron affinity
The energy change when an electron is added to a neutral atom in the gas phase.
- Oxidizing agent
A substance that tends to bring about oxidation by being reduced itself.
- Ionic halides
Compounds formed when Halogens react with metals, resulting in ionic bonds.
- Standard reduction potential
The measure of the individual half-cell voltage of a reduction reaction at standard conditions.
Reference links
Supplementary resources to enhance your learning experience.
