Filling of Principal Energy Levels (Periods 2 and 3)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Electron Configuration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll discuss how the filling of principal energy levels in Periods 2 and 3 leads to the unique properties of different elements. Let's start by looking at the electron configuration in these periods.

What exactly do we mean by electron configuration?

Good question! Electron configuration is the arrangement of electrons around the nucleus of an atom. In Periods 2 and 3, we fill the 1s, 2s, and 2p orbitals in a specific order, following the Aufbau principle. Can anyone list the first few elements in Period 2 with their configurations?

Sure! Lithium is 1s² 2s¹, Beryllium is 1s² 2s², and Boron is 1s² 2s² 2p¹.

Exactly! As we add electrons, we see increasing atomic numbers, which influences their chemical behavior.

How does this filling affect their properties?

Great question! The way electrons fill these shells helps explain trends in ionization energy and atomic radii. We'll explore more about that in our next discussion!
Trends in Period 2
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive deeper into Period 2 configurations. Why do you think atomic size decreases as we go from Lithium to Neon?

Because we have more protons pulling the electrons in closer?

Exactly! This is known as Effective Nuclear Charge, or Z_eff. With each atom, protons increase but shielding doesn’t change much, leading to a stronger pull on the electrons.

And does this trend continue into Period 3?

Yes! We see similar filling with elements from sodium to argon, where atomic radius continues to decrease until Argon where it is minimized.

So increasing protons mean increasing attraction for all elements in a period?

Correct! It’s all about the balance between the nuclear charge and the shielding effect. Remember, it’s this balance that drives the trends in the periodic table.
Chemical Reactivity in Periods 2 and 3
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've explored the configurations, let's talk about reactivity. Why do elements in the same group react similarly?

It’s because they have the same number of valence electrons!

Exactly! In Period 2, elements like Lithium and Sodium have similar reactions with water, categorized by their valence electron count, which makes predicting behavior easier.

So, does this mean that Fluorine will be highly reactive?

Yes, Fluorine has a high electronegativity due to its electron configuration, pulling electrons in, leading to strong reactivity. Remember, the tendency to gain or lose electrons is defined by their electron configuration.

Interesting! So that’s like a pattern we can see across different periods!

Exactly! Patterns in electron filling provide insights into both the physical and chemical properties of the elements.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In Periods 2 and 3, elements progressively fill their valence shells — first the s-orbitals, followed by p-orbitals, leading to specific trends in atomic properties such as size and ionization energy. Understanding these filling patterns assists in predicting element behavior and chemical reactivity.
Detailed
Filling of Principal Energy Levels (Periods 2 and 3)
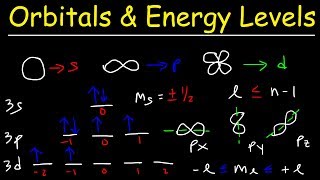
In this section, we analyze how the elements in Periods 2 and 3 of the Periodic Table fill their principal energy levels. The electron configurations for these periods illustrate that for Period 2, the electrons fill from 1s to 2s and then to 2p orbitals sequentially, whereas in Period 3, electrons follow a similar filling order (1s, 2s, 2p) before moving to 3s and then 3p.
Period 2 Electronic Configurations:
- Lithium (Li): 1s² 2s¹
- Beryllium (Be): 1s² 2s²
- Boron (B): 1s² 2s² 2p¹
- Carbon (C): 1s² 2s² 2p²
- Nitrogen (N): 1s² 2s² 2p³
- Oxygen (O): 1s² 2s² 2p⁴
- Fluorine (F): 1s² 2s² 2p⁵
- Neon (Ne): 1s² 2s² 2p⁶
Period 3 Electronic Configurations:
- Sodium (Na): [Ne] 3s¹
- Magnesium (Mg): [Ne] 3s²
- Aluminum (Al): [Ne] 3s² 3p¹
- Silicon (Si): [Ne] 3s² 3p²
- Phosphorus (P): [Ne] 3s² 3p³
- Sulfur (S): [Ne] 3s² 3p⁴
- Chlorine (Cl): [Ne] 3s² 3p⁵
- Argon (Ar): [Ne] 3s² 3p⁶
As electrons fill these subshells, certain trends become apparent, showing slight irregularities caused by electron-electron repulsions. These filling patterns are not merely a linear progression; they provide insight into the properties of the elements and their chemical behavior, setting the stage for understanding periodic trends such as ionization energy and atomic radius.
Youtube Videos
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Filling in Period 2
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Period 2: 1s (filled for He); valence electrons occupy 2s and then 2p orbitals sequentially.
- Li: 1s² 2s¹
- Be: 1s² 2s²
- B: 1s² 2s² 2p¹
- C: 1s² 2s² 2p²
- N: 1s² 2s² 2p³
- O: 1s² 2s² 2p⁴
- F: 1s² 2s² 2p⁵
- Ne: 1s² 2s² 2p⁶
Detailed Explanation
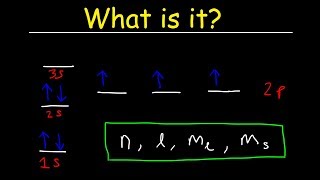
In Period 2 of the periodic table, elements start filling the 2s and then the 2p orbitals after the 1s orbital is filled. The first element, Lithium (Li), has three electrons. Two electrons fill the 1s orbital, and one electron goes into the 2s orbital. As you proceed through the period, each element adds additional electrons to the 2s and then 2p orbitals according to the Aufbau principle, which states that electrons occupy the lowest energy orbitals first. The last element, Neon (Ne), has filled all available orbitals in this period with a total of 10 electrons.
Examples & Analogies
Think of Period 2 like filling up a set of boxes in a storage shed. The first box represents the 1s orbital, which can hold 2 items (electrons). After that box is full, you move to the next box (the 2s orbital), and then to the bigger set of compartments (the 2p orbitals). Each time you add something to the boxes, it becomes more packed, and you can't add more until one of the boxes is filled.
Filling in Period 3
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Period 3: 1s, 2s, 2p (core), then valence 3s → 3p.
- Na: [Ne] 3s¹
- Mg: [Ne] 3s²
- Al: [Ne] 3s² 3p¹
- Si: [Ne] 3s² 3p²
- P: [Ne] 3s² 3p³
- S: [Ne] 3s² 3p⁴
- Cl: [Ne] 3s² 3p⁵
- Ar: [Ne] 3s² 3p⁶
Detailed Explanation
In Period 3, the filling process continues with the 3s and then the 3p orbitals being filled. Sodium (Na) has 11 electrons, which means that after filling the first two periods, you now start filling the 3s level with 1 electron. As you progress across the period, each subsequent element adds electrons to either 3s or 3p orbitals. By the time you reach Argon (Ar), the period is completed with a total of 18 electrons filling the orbitals. The notation '[Ne]' signifies that the electron configuration takes into account previous noble gas, Neon (Ne), which is already filled.
Examples & Analogies
Consider Period 3 like constructing a new floor in an office building. Each room represents a subshell that you fill with occupants (electrons). You start with the first room (3s) and add people (electrons) one by one. When that room is full, you move on to the next set of offices (3p). Just like a building fills up with tenants, these orbitals fill with electrons until they reach capacity.
Electron Configuration Patterns
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As electrons fill subshells, subtle variations in electron–electron repulsions yield slight irregularities in trends (e.g., the O vs. N ionization energy anomaly).
Detailed Explanation
Even though generally, trends in ionization energies and other properties are predictable based on the filling of orbitals, there can be irregularities. For instance, the ionization energy of oxygen is unexpectedly lower than that of nitrogen due to the electron-electron repulsion in the paired p-orbitals in oxygen. This disturbs the expected trend, demonstrating that while we can establish rules, the interactions between electrons must also be taken into account.
Examples & Analogies
Imagine a crowded party where everyone is trying to dance in a small space. If too many couples are in close proximity, they might bump into each other and create frustration, making it harder for one to leave and dance freely. Thus, at times, the energy needed to 'leave the dance floor' (ionization energy) might be lower for some than expected due to these interactions, just like the electron pairs in oxygen.
Key Concepts
-
Electron Configuration: The filling of orbitals in order according to specified rules, leading to the formation of elements with specific properties.
-
Effective Nuclear Charge (Z_eff): The net positive charge experienced by electrons from the nucleus, impacting radius and reactivity.
-
Trends Across Periods: Elements show clear trends in properties such as atomic size, ionization energy, and chemical reactivity as you move across a period.
Examples & Applications
For instance, the electron configuration for Oxygen is 1s² 2s² 2p⁴, suggesting it will gain two electrons to achieve a stable octet.
In Period 3, Sodium and Magnesium show similar reactivities, forming ionic compounds with nonmetals.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In Period Two, electrons will pack, from 1s to 2s, no lack!
Stories
Once upon a time in an atom, the electrons lined up, filling the orbits to finally make it stable, creating families that behave together like noble gas friends.
Memory Tools
Li Be B C N O F Ne- Learn Beginning Chemistry Neatly in Period 2!
Acronyms
PET
Periods Electron Trends - Remember
Flash Cards
Glossary
- Electron Configuration
The arrangement of electrons in an atom's orbitals.
- Effective Nuclear Charge (Z_eff)
The net positive charge experienced by valence electrons in an atom.
- Atomic Radius
The distance from the nucleus to the outermost shell of an electron in an atom.
- Chemical Reactivity
The tendency of a substance to undergo chemical changes in a given environment.
Reference links
Supplementary resources to enhance your learning experience.
