Periodic Trends in Atomic Properties
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Atomic Radius
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll explore the atomic radius. Can anyone tell me how we define it?

It's the distance from the nucleus to the outermost electron.

Exactly! More specifically, we define it as the covalent radius, which is half the distance between two bonded nuclei. Now, what happens to the atomic radius as you move down a group?

It increases because there are more energy levels.

Right! As we add more principal quantum levels, the electrons are farther from the nucleus, leading to a larger atomic radius despite the increasing nuclear charge. How about across a period?

The atomic radius decreases because of the increased effective nuclear charge.

Great! The higher charge pulls the electrons closer, making the atom smaller. Remember the acronym 'ACE' – Atomic radius decreases across periods!

Got it, ACE for Across Periods - decreases!

Let's summarize: atomic radius increases down a group and decreases across a period due to effective nuclear charge and shielding effects.
Ionization Energy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss ionization energy. Who can define it for us?

It's the energy needed to remove an electron from an atom.

Very well! How does this change when moving across a period?

It increases because the atomic radius decreases.

Exactly! As you move across, Z_eff increases, requiring more energy to remove an electron. What about going down a group?

It decreases because the electrons are further away from the nucleus.

Correct! To remember this, think of 'IE decreases Down': as we go down, electrons are shielded more. Can someone explain why there are jumps in ionization energy?

Jumps happen after removing a core electron; it becomes much harder!

Exactly right! Let’s wrap up: ionization energy increases across a period and decreases down a group, tied closely to atomic structure.
Electronegativity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's turn to electronegativity. Who can say what it measures?

It's how strongly an atom attracts electrons in a bond.

Yes! How does electronegativity change across a period?

It increases because the atomic radius decreases.

Perfect! And down a group?

It decreases because the electrons are further from the nucleus.

Exactly! Remember the saying 'Electronegativity Increases Across, Decreases Down!' What implications does this have for bond types?

It helps predict whether a bond is ionic or covalent based on differences in electronegativity!

Great connections! To summarize: electronegativity increases across a period and decreases down a group, which is essential for understanding chemical bonding.
Electron Affinity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s examine electron affinity. What does this refer to?

It's the energy change when an electron is added to an atom.

That's right! Is electron affinity generally more exothermic across a period or less?

More exothermic across a period, except in some groups like noble gases.

Good catch! What about down a group?

It becomes less exothermic because of the additional distance from the nucleus.

Exactly! Let's make this memorable: 'EA Exothermic Across, Endothermic Down!' Remember that noble gases have positive affinities too because gaining an electron is unfavorable for them.

Got it, that makes sense!

Wonderful! Let’s summarize: electron affinity typically becomes more exothermic across a period and less down a group, with exceptions!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses how atomic radius, ionic radius, ionization energy, electron affinity, electronegativity, and metallic vs. nonmetallic character change with increasing atomic number. It emphasizes the roles of effective nuclear charge and shielding in influencing these periodic trends.
Detailed
Periodic Trends in Atomic Properties
This section examines the systematic variations in atomic properties as exhibited in the periodic table, significantly influenced by effective nuclear charge (Z_eff) and the shielding effect. When elements are organized by increasing atomic number, they reveal consistent trends in multiple properties. The discussed properties include:
1. Atomic Radius
- Definition: The atomic radius, primarily characterized by the covalent radius (half the bond length between two identical atoms), is critical for understanding atomic size. Other types include van der Waals radius and metallic radius.
- Trend Down a Group: The atomic radius increases as one moves down a group due to the increment in principal quantum numbers and increased electron shielding.
- Trend Across a Period: Moving left to right, atomic radius decreases owing to rising Z_eff attracting electrons more strongly.
2. Ionic Radius
- Cationic Radii: Cations are smaller than their neutral counterparts because they lose electrons, resulting in reduced electron-electron repulsions.
- Anionic Radii: Conversely, anions are larger since they gain electrons, increasing repulsions without a corresponding increase in nuclear charge.
- Isoelectronic Series: Among isoelectronic ions, the size is inversely related to nuclear charge.
3. Ionization Energy
- Definition: The energy required to remove the most loosely bound electron. The first ionization energy (IE₁) indicates the energy needed for the first electron removal, and subsequent energies showcase increased difficulty with additional removals.
- Trend Across a Period: Increases left to right owing to rising Z_eff and smaller atomic radii.
- Trend Down a Group: Decreases downwards due to larger atomic sizes and increased shielding.
- Successive Ionization Energies: Each subsequent ionization energy is greater due to the increased positive charge of the ion after each electron removal.
4. Electron Affinity
- Definition: It measures the energy change when an electron is added to a neutral atom. Generally more exothermic across a period but with exceptions in specific groups, particularly noble gases.
- Trend Down a Group: Electron affinity becomes less exothermic down a group.
5. Electronegativity
- Definition: The ability of an atom to attract electrons in a bond.
- Trends: Increases across a period and decreases down a group, with implications for bond type predictions.
6. Metallic and Nonmetallic Character
Elements with low ionization energy and electronegativity are more metallic, found on the left side of the periodic table. In contrast, nonmetals exhibit these qualities as you progress to the right.
An understanding of these trends not only assists in grasping the nature of elemental reactivity and interactions but is foundational for delving deeper into chemical behavior in subsequent sections.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Periodic Trends
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When elements are arranged in order of increasing atomic number, certain physical and chemical properties show regular, predictable variation. These periodic trends arise primarily due to two factors:
- Effective Nuclear Charge (Z_eff)
- Shielding (Screening) Effect
Understanding how Z_eff and shielding vary helps explain why atomic radii get smaller across a period but larger down a group, why ionization energies increase across a period, and so on.
Detailed Explanation
Periodic trends refer to predictable patterns in atomic and ionic properties, such as atomic radius or ionization energy, based on an element's placement in the periodic table. The two main factors that affect these trends are Effective Nuclear Charge (Z_eff), which is the net positive charge felt by valence electrons, and the Shielding Effect, which is the reduction in attractive force between the nucleus and outer electrons due to the presence of inner electrons. For instance, as you move across a period from left to right, the Z_eff increases because protons are added to the nucleus while the number of inner shell electrons remains roughly the same. This results in stronger attraction for the outer electrons, causing a decrease in atomic radius. Conversely, as you go down a group, additional energy levels are added, resulting in increased atomic radius despite increased nuclear charge.
Examples & Analogies
Think of a crowded room where the number of people (representing protons) is increasing, but the size of the room (representing shielding from inner electrons) stays the same. As more people enter, it becomes harder for you to move freely because the room is getting more crowded. This represents the increasing Z_eff across a period. Conversely, if you're stepping from a small room to a bigger hall (representing moving down a group), even though more people are still coming in, you feel less crowded because you have more space around you (increased atomic radius).
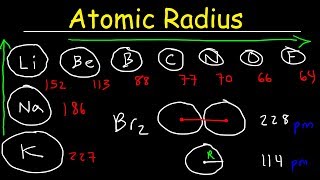
Atomic Radius
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Atomic Radius
Definition
- Atomic radius can be conceived in multiple ways (covalent radius, van der Waals radius, metallic radius, etc.), but for periodic trends we focus on the covalent radius (half the distance between the nuclei of two identical atoms bonded by a single covalent bond).
- For noble gases (which do not form stable diatomic molecules) and for metals in the metallic lattice, the van der Waals radius or metallic radius is used, respectively.
Trend Down a Group
- As you descend a group, the principal quantum number (n) of the valence shell increases by one each row (e.g., n = 2 for Li–Ne, n = 3 for Na–Ar, n = 4 for K–Kr).
- Each additional energy level lies farther from the nucleus; although nuclear charge (Z) also increases, the inner electrons increasingly shield outer electrons from the nucleus.
- Result: Atomic radius increases down a group.
Trend Across a Period
- Moving left to right across a period, electrons fill the same principal energy level (constant n).
- The number of protons in the nucleus increases by one with each successive element; each new valence electron experiences a higher effective nuclear charge (Z_eff).
- Although shielding by inner electrons remains essentially constant across a period, the increasing Z_eff draws the valence electrons closer to the nucleus.
- Result: Atomic radius decreases from left to right across a period.
Detailed Explanation
The atomic radius is a measure of the size of an atom, typically determined by the distance between the nuclei of two bonded atoms. It can vary depending on the context (as in covalent, van der Waals, or metallic radii). Trends in atomic radius show that as one moves down a group in the periodic table, the atomic radius increases. This happens because with each added row, electrons occupy higher energy levels, which are further away from the nucleus, and although the nuclear charge increases, the additional inner electrons create shielding that reduces the effective attraction on the outer electrons. Conversely, as you move across a period (left to right), the atomic radius decreases. This is because more protons are added to the nucleus, which increases the nuclear charge without adding new electron shells, effectively pulling the electrons closer to the nucleus and reducing the radius.
Examples & Analogies
Imagine a Ferris wheel (representing the nucleus) with people (electrons) sitting on the edge. At first, when there are only a few people, they can sit far apart (indicating a larger atomic radius). As more people join, they have to sit closer together to fit on the wheel (representing a decrease in atomic radius across a period). When you evaluate a taller Ferris wheel (for larger atoms lower in a group), although there are more people, they now have a much larger area to spread out in terms of height (indicating a larger atomic radius down a group).
Ionic Radius
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ionic Radius
Cationic Radii
- When an atom loses one or more electrons to form a cation, the resulting positive ion has fewer electron-electron repulsions and sometimes loses an entire valence shell if the highest principal quantum number becomes vacant.
- The nuclear attraction remains the same (same number of protons) but is now distributed over fewer electrons; thus, the electrons move closer to the nucleus.
- Result: Cationic radius is smaller than the atomic radius of the neutral atom.
Anionic Radii
- When an atom gains electrons to form an anion, electron-electron repulsions increase in the valence shell(s), and there is no corresponding increase in nuclear charge to offset this repulsion.
- This causes the electron cloud to expand.
- Result: Anionic radius is larger than the atomic radius of the neutral atom.
Isoelectronic Series
- In an isoelectronic series (ions having the same electron configuration), the ion with more protons (higher nuclear charge) is smallest, because greater positive charge draws electrons closer.
- Example: For O²⁻, F⁻, Na⁺, Mg²⁺, and Al³⁺ (all with 10 electrons), Al³⁺ (13 protons) has the smallest radius; O²⁻ (8 protons) has the largest.
Detailed Explanation
The ionic radius refers to the size of an ion. When atoms lose or gain electrons to form cations and anions, respectively, their radius changes. For cations, losing electrons decreases electron-electron repulsion and allows the remaining electrons to be pulled closer to the nucleus, resulting in a smaller radius compared to the neutral atom. For anions, gaining electrons increases electron-electron repulsion in the outer shell(s) without a corresponding increase in positive charge from the nucleus, causing the radius to expand. Additionally, in an isoelectronic series of ions, those with a higher number of protons will have a smaller ionic radius due to stronger effective nuclear charge.
Examples & Analogies
Consider a crowded bus where everyone is standing close to each other due to limited space (representing a cation). If someone leaves the bus, the remaining people can stand slightly farther apart, creating more room (a smaller size). Now, think of a balloon that you are inflating (the anion); as you add air (electrons), the balloon expands. If you keep adding air, it becomes more inflated due to repulsion between air molecules inside it, making it larger (expand, showing a larger anionic radius).
Ionization Energy
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ionization Energy
Definition
-
First ionization energy (IE₁): The energy required to remove the highest-energy (most loosely bound) electron from a gaseous atom in its ground state, forming a cation with a +1 charge:
X(g) ⟶ X+(g) + e− ΔE=IE₁. - Second ionization energy (IE₂): Energy needed to remove a second electron from the +1 cation, and so on.
Trend Across a Period
- Increases left → right across a period:
- Nuclear charge increases, atomic radius decreases, Z_eff increases → more energy is needed to remove an electron.
- Example: Li (IE₁ ≈ 520 kJ/mol), Be (IE₁ ≈ 900 kJ/mol), … to Ne (IE₁ ≈ 2080 kJ/mol).
Trend Down a Group
- Decreases top → bottom down a group:
- Atomic radius increases, outer electrons are farther from the nucleus and more effectively shielded by inner shells → less energy required to remove an electron.
- Example: Li (IE₁ ≈ 520 kJ/mol), Na (IE₁ ≈ 496 kJ/mol), K (IE₁ ≈ 419 kJ/mol).
Successive Ionization Energies
- Each successive ionization energy (IE₂, IE₃, etc.) is greater than the previous, because removing an electron from an increasingly positive ion requires more energy.
- Large jumps occur once the electron removed is from a noble‐gas‐like configuration.
Anomalies: Subshell Effects
- Small dips in the general trend appear between Group 2 → 13 (Be → B, Mg → Al) and Group 15 → 16 (N → O, P → S) due to:
- B and Al have an electron in a higher-energy p-subshell, which is easier to remove than an electron from a filled s-subshell (Be, Mg).
- O and S have paired electrons in a p-orbital, causing higher electron-electron repulsion than the singly occupied p-orbitals of N and P.
Detailed Explanation
Ionization energy is the energy required to remove an electron from an atom in its gaseous state. The first ionization energy refers to the removal of the most loosely bound electron, while successive ionization energies involve removing additional electrons. Trends in ionization energy show that as one moves across a period from left to right, ionization energy typically increases due to rising nuclear charge and decreasing atomic radius, which leads to better attraction between the nucleus and electrons. In contrast, as you go down a group, ionization energy decreases because the outer electrons are further from the nucleus and shielded by more inner electrons, resulting in less energy needed to remove them. Additionally, successive ionization energies show an increasing pattern, with significant jumps occurring when moving to remove electrons from inner shells.
Examples & Analogies
Imagine a game where you have to throw a ball (electron) as far as possible from a growing crowd (nucleus). The harder it is to throw the ball (higher ionization energy), the more people (protons) are crowding around as you progress through the game (moving across a period). If you were to drop your ball in a crowd that also becomes larger (moving down a group) but further away, you’ll find it easier to throw the ball away since fewer people are playing the game (decreasing ionization energy).
Electron Affinity
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Electron Affinity
Definition
-
Electron affinity (EA): The energy change (often released) when an electron is added to a gaseous atom, forming an anion:
X(g) + e− ⟶ X−(g) ΔE=EA. - Generally reported as the negative of ΔE if energy is released (exothermic process) or positive if energy must be absorbed (endothermic).
Trend Across a Period
- Generally becomes more exothermic (more negative) left → right across a period:
- Higher Z_eff and smaller atomic radius → the added electron experiences stronger attraction and releases more energy.
- Exceptions: Group 2 (Be, Mg) and Group 15 (N, P) elements have less negative EA than their neighbors.
Trend Down a Group
- Generally becomes less exothermic (less negative) top → bottom down a group:
- Additional electron is added to a higher principal shell farther from the nucleus → less energy release.
- Noble gases have positive (endothermic) EA because adding an electron forces entry into the next shell, which is energetically unfavorable.
Subtle Points
- Some values are endothermic (positive EA), notably Be, N, Mg, and noble gases. In these cases, it requires energy input to force an extra electron into a half-filled or filled orbital.
- Within the halogens (Group 17), Cl has a slightly more exothermic EA than F because the small size of F causes greater electron–electron repulsion when the second electron enters the 2p orbital.
Detailed Explanation
Electron affinity measures the energy change that occurs when an electron is added to an atom in the gas phase. This energy change can be exothermic (releasing energy, indicated by a negative value) or endothermic (absorbing energy, indicated by a positive value). Trends across a period show that as you move from left to right, electron affinity typically becomes more exothermic because increased effective nuclear charge and decreased atomic radius create a stronger attraction between the added electron and the nucleus. However, there are exceptions to this trend, particularly with Group 2 and Group 15 elements. Down a group, electron affinity generally becomes less exothermic due to increased distance from the nucleus and decreased attraction. For noble gases, the process is endothermic, as adding an electron disrupts their stable electronic configuration.
Examples & Analogies
Think of a person catching a thrown baseball (the added electron). The closer the person is to the thrower (the nucleus), the easier it is to catch the ball (stronger attraction and more energy is released). As you move further away (down a group), catching it becomes harder (the attraction diminishes), so the thrower has to expend more energy, indicating less energy is release when catching the ball. At some point, it becomes impossible to catch any more baseballs (like the noble gases)—instead of energy being released, they need extra help to bring another ball into their space (positive EA).
Electronegativity
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Electronegativity
Definition
- Electronegativity is a dimensionless measure of an atom’s ability to attract electrons toward itself in a covalent bond.
- The most widely used scale is the Pauling scale, where fluorine is assigned 4.0 (highest) and values decrease from there.
Trend Across a Period
- Increases left → right:
- As Z_eff increases and atomic radius decreases, atoms more strongly attract bonding electrons.
- Example: Li ≈ 0.98, Be ≈ 1.57, B ≈ 2.04, C ≈ 2.55, up to F ≈ 3.98.
Trend Down a Group
- Decreases top → bottom:
- The valence electrons occupy orbitals farther from the nucleus and experience more shielding → lower attraction for bonding electrons.
- Example: F ≈ 3.98, Cl ≈ 3.16, Br ≈ 2.96, I ≈ 2.66.
Applications
- Predicting bond polarity: Δχ = |χ_A – χ_B|
- Δχ < 0.5 → nonpolar covalent
- 0.5 ≤ Δχ < 1.7 → polar covalent
- Δχ ≥ 1.7 → predominantly ionic.
Detailed Explanation
Electronegativity is a property that measures how strongly an atom can attract electrons in a chemical bond. The Pauling scale is commonly used, with fluorine being the most electronegative element assigned a value of 4.0. Trends in electronegativity show that as you move across a period from left to right, electronegativity increases due to rising effective nuclear charge and decreasing atomic radius, which enhance an atom's ability to attract bonding electrons. Conversely, moving down a group results in decreasing electronegativity because increasing distance and shielding reduce an atom's ability to attract electrons. This concept is essential for predicting bond types between elements—whether they form covalent, polar covalent, or ionic bonds based on electronegativity differences.
Examples & Analogies
Imagine a group project where some students are trying to grab pens from a box (electrons). The closer a student (atom) is to the box (nucleus), the more eager they are to get those pens (high electronegativity). As you move deeper in the classroom (down a group), students are farther away from the box and kind of shielded by their peers (more inner electrons), so they show less interest (lower electronegativity). By comparing how many pens two students try to grab, you can predict whether they will work well together or if they'll fight over the pens (bond polarity based on their electronegativity differences).
Metallic and Nonmetallic Character
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Metallic and Nonmetallic Character
- Elements with low ionization energies and low electronegativities tend to lose electrons easily and exhibit metallic behaviour (malleability, ductility, conductivity). These are found on the left and center of the Periodic Table.
- Elements with high ionization energies and high electronegativities tend to gain electrons or share electrons in covalent bonds and exhibit nonmetallic behaviour (brittleness as solids, lack of metallic lustre, poor electrical conductivity). These are found on the right side of the Periodic Table (excluding noble gases, which are inert).
- Metalloids (B, Si, Ge, As, Sb, Te, Po) have intermediate properties and form a zigzag diagonal between metals and nonmetals.
Detailed Explanation
Metallic and nonmetallic characters refer to the general behavior of elements based on their ability to lose or gain electrons. Metals, located on the left and center of the periodic table, have low ionization energies and electronegativities, making them prone to losing electrons; thus, they are malleable and good conductors of heat and electricity. In contrast, nonmetals found on the right side exhibit high ionization energies and electronegativities, making them more likely to gain or share electrons than lose them, which leads to characteristics such as brittleness and less metallic luster. Metalloids have properties that fall between those of metals and nonmetals, demonstrating a mixture of both characteristics.
Examples & Analogies
Think of a lightweight, flexible rubber band (nonmetal) versus a sturdy metal wire (metal). The rubber can bend but will snap if you stretch it too far (high ionization energy and nonmetallic properties), while the metal wire can be twisted or stretched without breaking (low ionization energy and metallic properties). Just like how we can use different materials for various functions (like rubber for flexibility and metal for strength), elements in the periodic table have their own roles based on their metallic or nonmetallic character.
Key Concepts
-
Atomic radius increases down a group and decreases across a period.
-
Cations are smaller than their neutral atoms; anions are larger.
-
Ionization energy increases across a period and decreases down a group.
-
Electron affinity generally becomes more exothermic across periods and less down groups.
-
Electronegativity increases across a period and decreases down a group.
Examples & Applications
In an isoelectronic series like Na⁺, Mg²⁺, and F⁻, the ionic radii decrease as the nuclear charge increases.
The ionization energy of sodium (IE₁ ≈ 496 kJ/mol) is lower than that of neon (IE₁ ≈ 2080 kJ/mol) since Ne has a filled valence shell.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Atomic size goes up with layers, down a group will call for prayers!
Stories
Imagine electrons as children in a playground. As new kids (energy levels) join, the playground (atomic radius) gets larger. But in a compact park (period), the rules (effective nuclear charge) keep them closer together.
Memory Tools
EIE: Electronegativity Increases Eastward.
Acronyms
ICE
Increase across
Decrease down for electron affinity.
Flash Cards
Glossary
- Atomic Radius
Half the distance between nuclei of two bonded atoms of the same element.
- Cation
A positively charged ion.
- Anion
A negatively charged ion.
- Ionization Energy (IE)
Energy required to remove an electron from a gaseous atom or ion.
- Electron Affinity (EA)
Energy change when an electron is added to a gaseous atom.
- Electronegativity (χ)
Tendency of an atom to attract electrons in a chemical bond.
- Effective Nuclear Charge (Z_eff)
Net positive charge experienced by valence electrons after accounting for shielding by inner electrons.
- Shielding (Screening) Effect
Reduction in attraction between nucleus and outer electrons caused by presence of inner electrons.
- Periodicity
Recurring trends in properties of elements arranged by increasing atomic number.
Reference links
Supplementary resources to enhance your learning experience.
