Strong and Weak Acids/Bases
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Defining Strong Acids and Bases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re diving into the world of acids and bases! First, let’s start with strong acids and bases. Can anyone tell me how we define a strong acid?

Is it one that completely dissociates in water?

Exactly! Strong acids, like hydrochloric acid, dissociate nearly 100% in solution, releasing H⁺ ions. Now, what about strong bases?

Strong bases also dissociate completely, like sodium hydroxide?

Correct! They produce OH⁻ ions in water. Remember the acronym 'CHN’ for common strong acids: Chloric, Hydrobromic, Nitric! Let’s take a quick quiz. Can anybody give me an example of each?

Hydrochloric acid for strong acid and sodium hydroxide for strong base.

Great job! So, strong acids and bases fully dissociate in water, making them robust players in chemical reactions. Let’s summarize: Strong acids include HCl, HNO₃, and sulfuric acid, while strong bases include NaOH and KOH.
Exploring Weak Acids and Bases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s shift our focus to weak acids and bases. Can anyone explain what makes an acid 'weak'?

Weak acids only partially dissociate in water, right?

Correct! For instance, acetic acid (CH₃COOH) doesn’t fully dissociate, and we measure its strength using the acid dissociation constant, Ka. What do we call the ions produced when it dissolves?

H⁺ and acetate ions!

Well done! And similarly, what about weak bases?

They partially accept protons in solution, like ammonia.

Very good! An important relationship to remember is that the strength of a weak acid is related to its Kb value for its conjugate base. Let’s summarize: Weak acids do not fully dissociate, which aids in understanding their behavior in equilibrium.
Understanding Polyprotic Acids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, let’s talk about polyprotic acids. Anyone know what that term means?

They are acids that can donate more than one proton!

Exactly! Examples include sulfuric acid and carbonic acid. What can you tell me about their dissociation steps?

They dissociate in stages, so each step has its own Ka value.

Correct! The first dissociation is usually the strongest, and we often consider it first when assessing their strength. Don't forget that these acids can also produce amphoteric ions. Let's summarize: Polyprotic acids dissociate stepwise with decreasing strength.
Acid and Base Strength Relationships
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s explore the relationships between different constants. Who can tell me how Ka, Kb, and Kw are interrelated?

Ka times Kb equals Kw?

Exactly! This relationship is vital for assessing the strength of conjugate acid-base pairs. If Ka is large, what does that imply about Kb for the conjugate base?

Kb would be small since the acid is strong!

Correct! It’s important to remember the context in which we apply these constants, particularly when calculating pH or pOH. Let’s summarize: the relationship between Ka and Kb is critical for understanding acid-base strength.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
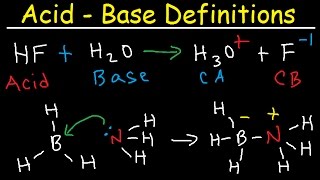
In this section, we explore strong and weak acids and bases, outlining their defining characteristics, common examples, and the mathematical principles governing their behavior in solution. The section differentiates between complete and partial dissociation, explains acid-base dissociation constants, and introduces concepts related to polyprotic acids and amphoteric substances.
Detailed
Strong and Weak Acids/Bases
This section provides a detailed examination of acids and bases, categorizing them by their dissociation in water. Strong acids and bases are defined as substances that dissociate completely in an aqueous solution, whereas weak acids and bases partially dissociate.
Strong Acids and Bases
Strong acids, such as Hydrochloric acid (HCl) and Sulfuric acid (H₂SO₄), dissociate nearly 100% in water, releasing hydrogen ions (H⁺) and forming their conjugate bases. Common strong bases include Sodium hydroxide (NaOH) and Calcium hydroxide (Ca(OH)₂), which fully dissociate to produce hydroxide ions (OH⁻).
Weak Acids and Bases
In contrast, weak acids, such as Acetic acid (CH₃COOH) and Formic acid (HCOOH), do not fully dissociate, which leads to an equilibrium between the undissociated acid and its ions. The concept of the acid dissociation constant (Ka) quantifies the strength of weak acids, while weak bases, like Ammonia (NH₃), can be analyzed through their base dissociation constant (Kb).
Key Relationships
For a conjugate acid-base pair, the relationships between Ka, Kb, and Kw provide key insights into their relative strengths. For polyprotic acids, which possess more than one proton that can dissociate, the first dissociation is often the strongest. Amphoteric substances, which can act as either acids or bases, are also discussed, demonstrating the versatility in acid-base chemistry.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
3.1 Strong Acids and Bases
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
3.1 Strong Acids and Bases
3.1.1 List of Common Strong Acids
- Hydrochloric acid (HCl)
- Hydrobromic acid (HBr)
- Hydroiodic acid (HI)
- Sulfuric acid (H₂SO₄) – the first proton is strong; the second proton (HSO₄ minus → H plus + SO₄² minus) is considered a strong acid if concentration is high, but at moderate dilutions the second dissociation is weak.
- Perchloric acid (HClO₄)
- Nitric acid (HNO₃)
Behavior:
- Virtually 100% of the acid molecules dissociate into H plus and their conjugate base in water.
Detailed Explanation
Strong acids are substances that completely dissociate in water, meaning they release all of their available hydrogen ions (H+) into the solution. The common strong acids listed, such as hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), have this characteristic. In the case of sulfuric acid, while its first dissociation is complete, the second dissociation may not be fully complete depending on the concentration. Since strong acids release a high concentration of protons, they are also very effective at lowering the pH of solutions, making them very acidic.
Examples & Analogies
Think of a strong acid like a very forceful sprayer. When you pull the trigger, it releases all its water immediately, representing the rapid dissociation of strong acids. Conversely, a weak acid would be like a squirt bottle that requires multiple presses to release the same amount of water, illustrating how weak acids do not completely dissociate.
3.1.2 List of Common Strong Bases
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
3.1.2 List of Common Strong Bases
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Lithium hydroxide (LiOH)
- Calcium hydroxide (Ca(OH)₂) – only slightly soluble but highly dissociated in the dissolved portion.
- Barium hydroxide (Ba(OH)₂) – similar to Ca(OH)₂.
- Strontium hydroxide (Sr(OH)₂)
- Cesium hydroxide (CsOH)
Behavior:
- Dissociate completely to give OH minus and the metal cation. (Solubility can be limiting factor for some metal hydroxides.)
Detailed Explanation
Strong bases are substances that completely dissociate in water to produce hydroxide ions (OH-), thereby increasing the basicity of the solution. Common strong bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH). Just as with strong acids, the key feature of strong bases is their ability to provide a high concentration of OH- ions in a solution, resulting in a higher pH, indicating a basic environment.
Examples & Analogies
Imagine a strong base as a powerful cleaning solution that can instantly neutralize acid spills. As it comes into contact with acids, it completely transforms the solution, much like how a strong base completely dissociates into hydroxide ions, effectively neutralizing the acidity.
3.1.3 pH Calculation for Strong Acids/Bases
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
3.1.3 pH Calculation for Strong Acids/Bases
- For a solution of a strong acid with concentration C, [H plus] = C (assuming negligible contribution from water if C > 10⁻⁶).
- For a solution of a strong base with concentration C, [OH minus] = C (again, ignoring water).
- At 25 °C, pH + pOH = 14.
Example 1: A solution is prepared by dissolving 0.250 mol of HCl in 1.00 L of water.
- [H plus] = 0.250 M → pH = – log₁₀ (0.250) = 0.60.
Example 2: A solution is prepared by dissolving 0.0200 mol of Ca(OH)₂ in 500 mL of water. Ca(OH)₂ dissociates into Ca² plus + 2 OH minus.
- Moles of Ca(OH)₂ = 0.0200 mol, volume = 0.500 L → 0.0400 M Ca(OH)₂.
- [OH minus] = 2 × 0.0400 M = 0.0800 M.
- pOH = – log₁₀ (0.0800) = 1.10 → pH = 14.00 – 1.10 = 12.90.
Detailed Explanation
Calculating the pH for strong acids and strong bases is straightforward because they completely dissociate. For a strong acid, the concentration of hydrogen ions [H+] equals the concentration of the acid. For example, if you have 0.250 M HCl, this means [H+] is also 0.250 M, leading to a pH of 0.60. Similarly, for strong bases, you calculate the concentration of hydroxide ions [OH-] and compute the pH using the relationship that pH + pOH = 14.
Examples & Analogies
Imagine you have a full jar of marbles, each representing a hydrogen ion in a strong acid. If you know exactly how many marbles (H+) there are in the jar (the concentration), you can quickly determine how much acid the jar holds (pH). With strong bases, think of it as having an empty jar fillable by a hose (OH-); the faster you deliver more water, the higher the basicity, allowing you to easily measure how basic the solution is.
3.2 Weak Acids and Bases
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
3.2 Weak Acids and Bases
3.2.1 Acid Dissociation Constant (Ka)
For a weak acid HA in water:
HA ⇌ H plus + A minus
- Ka = ([H plus] × [A minus]) ÷ [HA] at equilibrium.
We denote the initial concentration of HA as C₀, and assume initially [H plus] and [A minus] are zero (unless acid or base is already present). At equilibrium, let [H plus] = x, [A minus] = x, and [HA] = C₀ – x. Then:
Ka = x × x ÷ (C₀ – x) = x² ÷ (C₀ – x)
Approximations:
- If Ka is significantly smaller than C₀ (for example, Ka < 10⁻² and C₀ > 0.01), then x will be small compared to C₀, so C₀ – x ≈ C₀, and we use x ≈ sqrt(Ka × C₀).
- After finding x, calculate pH = – log₁₀ (x).
Percent Ionization:
- Percent ionization = ([A minus] at equilibrium ÷ C₀) × 100% = (x ÷ C₀) × 100%. As C₀ increases, percent ionization decreases.
Detailed Explanation
Weak acids do not completely dissociate in solution. The degree of dissociation can be quantified using the acid dissociation constant (Ka). To calculate the pH of a weak acid, you first determine the equilibrium concentrations of the ions formed based on the initial concentration and Ka. A key factor in this calculation is the assumption that x (the amount that dissociates) is small compared to C₀. The percent ionization provides insights into how much of the weak acid actually dissociates into ions, which is generally less than 100%.
Examples & Analogies
Consider a weak acid as someone who is shy in a group. They might only participate a little (dissociate partially) rather than jumping into every conversation (completely dissociate). The acid dissociation constant (Ka) represents how likely they are to join in the conversation; a higher Ka means they are more likely to participate, just like a weak acid that can still ionize effectively in solution.
Key Concepts
-
Strong Acids are fully dissociated in solution and show high conductivity.
-
Weak Acids only partially dissociate, establishing an equilibrium.
-
The dissociation constants Ka and Kb indicate the strengths of acids and bases, respectively.
-
Polyprotic acids dissociate in stages, each with its own Ka value.
Examples & Applications
HCl and HNO₃ are examples of strong acids that dissociate completely.
Acetic acid (CH₃COOH) is an example of a weak acid that only partially dissociates in solution.
Ammonia (NH₃) is a weak base that only partially accepts a proton.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
For acids hot and bold, strong ones are all told, they fully do dissolve, in water they evolve.
Stories
Once a weak acid met a strong acid. The strong acid boasted about fully dissolving in water while the weak acid expressed its struggles with partial dissociation. They both learned each had their unique roles in chemistry.
Memory Tools
Remember 'ACID' for strong acids: Acids Completely Ionize Dissolved.
Acronyms
Use 'BASE' for strong bases
Bases Always Supply Electrons.
Flash Cards
Glossary
- Strong Acid
An acid that dissociates completely in water, such as HCl or HNO₃.
- Weak Acid
An acid that only partially dissociates in water, like acetic acid (CH₃COOH).
- Strong Base
A base that completely dissociates in water, such as NaOH.
- Weak Base
A base that partially accepts protons in water, such as ammonia (NH₃).
- Polyprotic Acid
An acid that can donate more than one proton, like sulfuric acid (H₂SO₄).
- Amphoteric Substance
A substance that can act as either an acid or a base.
- Ka
The acid dissociation constant, a measure of the strength of an acid.
- Kb
The base dissociation constant, a measure of the strength of a base.
- Kw
The ion-product constant for water, equal to 1.0 × 10⁻¹⁴ at 25 °C.
Reference links
Supplementary resources to enhance your learning experience.
