Theories of Acids and Bases
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Arrhenius Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome everyone! Today, we're diving into the Arrhenius Theory of acids and bases. Can someone tell me what they think an Arrhenius acid is?

I think it’s something that produces H⁺ ions in water.

Exactly! An Arrhenius acid, like hydrochloric acid, increases hydrogen ion concentration when dissolved in water. Now, what about an Arrhenius base?

I believe it produces OH⁻ ions.

Correct! Sodium hydroxide, for example, dissociates in water to produce hydroxide ions. However, can anyone tell me a limitation of the Arrhenius Theory?

It only works in water and doesn't explain acid-base reactions that happen in other solvents.

That's right! Remember, Arrhenius Theory is strictly for aqueous solutions. Let’s sum up: Arrhenius acids increase H⁺ and bases increase OH⁻ in water, but the scope is limited!
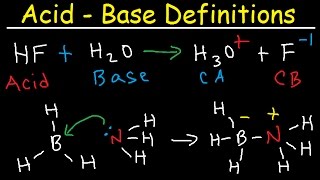
Brønsted-Lowry Theory and Conjugate Acid-Base Pairs
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's introduce the Brønsted-Lowry Theory. Who can explain what it states?

It says that an acid donates a proton, and a base accepts a proton.

Exactly! For example, when hydrochloric acid donates a proton to water, what happens?

HCl forms Cl⁻ and water becomes H₃O⁺.

Correct! Now, what can you tell me about conjugate acid-base pairs?

When an acid loses a proton, it becomes its conjugate base. And the base that accepts the proton becomes the conjugate acid.

Right! This relationship is vital in understanding how acid-base reactions work. Remember: donating and accepting protons leads to conjugate pairs!
Lewis Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's move on to the Lewis Theory. What distinguishes this theory from the previous ones?

It focuses on electron pairs instead of just protons.

Exactly! Lewis acids accept electron pairs and Lewis bases donate them. Can anyone provide an example of a Lewis acid?

BF₃ is a Lewis acid because it can accept electron pairs.

Perfect! And ammonium, NH₃, is a Lewis base due to its lone pair. Remember, this theory is much broader and can explain numerous reactions beyond simple proton exchanges. Great work today!
Comparison of Theories
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's recap what we learned about these theories. What are the key differences between Arrhenius, Brønsted-Lowry, and Lewis theories?

Arrhenius is limited to water, while Brønsted-Lowry covers more solvent scenarios. Lewis goes beyond proton transfer.

Excellent summary! Can you also tell me how these theories relate to each other?

Every Brønsted-Lowry acid is a Lewis acid, just like every Brønsted-Lowry base is a Lewis base.

Right again! Understanding these relationships is crucial. Let’s conclude with a final point: even if limits exist, these theories enrich our comprehension of acid-base chemistry!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides an overview of the four main theories for understanding acids and bases: Arrhenius theory, which focuses on ion production in water; Brønsted-Lowry theory, which emphasizes proton transfer; Lewis theory, which involves electron pair donation and acceptance; and conjugate acid-base pairs that illustrate the relationship between acids and their bases.
Detailed
Theories of Acids and Bases
Chemists have established several significant theories that define acids and bases over time. Each successive theory addresses the limitations of the previous one and enhances our understanding of acid-base chemistry:
1. Arrhenius Theory
- Definition: An Arrhenius acid produces hydrogen ions (H⁺) in aqueous solution, while an Arrhenius base produces hydroxide ions (OH⁻).
- Limitations: It applies only to aqueous solutions and cannot encompass all acid-base reactions outside of this scope.
2. Brønsted-Lowry Theory
- Definition: Brønsted-Lowry acids donate protons, and bases accept them. This theory extends beyond water and accommodates more chemical reactions.
- Conjugate Acid-Base Pairs: It establishes that every acid has a conjugate base and every base has a conjugate acid.
3. Lewis Theory
- Definition: A Lewis acid is an electron-pair acceptor, and a Lewis base is an electron-pair donor. This theory broadens acid-base chemistry to include non-proton interactions and interactions in non-aqueous solvents.
By studying these theories, we can understand the fundamentals of acid-base reactions and their implications in various chemical and biological systems.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Acid-Base Theories
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Over time, chemists have developed several models to describe what makes a substance an acid or a base. Each successive theory generalized and corrected limitations of its predecessor. In this section, we will study the four main classical theories: Arrhenius, Brønsted-Lowry, Lewis, and the concept of conjugate acid-base pairs.
Detailed Explanation
This introductory section emphasizes the evolution of acid-base theories in chemistry. Chemists identified that substances could behave as acids or bases in various ways, leading to the development of different models. Each model seeks to improve upon the previous one, addressing its limitations and providing a broader understanding of acid-base behavior.
Examples & Analogies
Think of these theories like different ways to explain how a car works. The Arrhenius theory might be like explaining a car solely based on its engine, while the Brønsted-Lowry theory expands this by considering how the car interacts with the road and other vehicles. Finally, the Lewis theory includes even more factors, like the fuel's chemical makeup, to explain performance.
Arrhenius Theory
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Definition:
- An Arrhenius acid is a substance that, when dissolved in water, increases the concentration of hydrogen ions (H⁺).
- An Arrhenius base is a substance that, when dissolved in water, increases the concentration of hydroxide ions (OH⁻).
Examples:
- Hydrochloric acid (HCl) in water dissociates to produce H⁺ and Cl⁻.
- Sodium hydroxide (NaOH) in water dissociates to produce Na⁺ and OH⁻.
Key Features:
- Limited to aqueous solutions.
- Explains acid-base behavior in terms of ions in water.
Limitations:
- Cannot explain acid-base reactions in nonaqueous solvents.
- Cannot classify substances like ammonia (NH₃) as a base.
Detailed Explanation
Arrhenius theory provides a specific definition of acids and bases based on their behavior in water. Arrhenius acids increase the concentration of H⁺ ions, while bases increase OH⁻ ions. However, its limitations include the inability to explain the behavior of these substances in non-solution contexts, such as reactions in solvents other than water.
Examples & Analogies
Think of Arrhenius acids and bases like a recipe for making lemonade. The lemon juice represents the acid, which contributes "sourness" (H⁺), and the sugar represents the "sweetness" (OH⁻). But this recipe only works when you have water (the solution); without it, you can't get lemonade, just like how these definitions only apply in an aqueous environment.
Brønsted-Lowry Theory
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Definition:
- A Brønsted-Lowry acid is a substance that donates a proton (H⁺) to another substance.
- A Brønsted-Lowry base is a substance that accepts a proton (H⁺) from another substance.
Conjugate Acid-Base Pairs:
- After donating a proton, an acid becomes its conjugate base.
- After accepting a proton, a base becomes its conjugate acid.
Detailed Explanation
The Brønsted-Lowry theory enhances our understanding of acids and bases by focusing on proton transfer. This means that the definition of acids and bases is not restricted to water and can include various chemical reactions, allowing us to classify substances based on their proton donation or acceptance.
Examples & Analogies
Imagine two friends passing a basketball during a game. The friend throwing the ball represents the acid donating a proton, and the friend receiving it is the base that accepts the proton. After the pass, the thrower now stands without the ball (conjugate base), while the receiver becomes more involved in the game (conjugate acid).
Lewis Theory
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Definition:
- A Lewis acid is an electron-pair acceptor.
- A Lewis base is an electron-pair donor.
Examples:
- Borane (BF₃) and Ammonia (NH₃): BF₃ accepts an electron pair from NH₃, forming a coordinate bond.
Detailed Explanation
The Lewis theory shifts the focus from protons to electron pairs, enabling a clearer explanation for a wider range of reactions that do not involve protons directly. It encompasses both Arrhenius and Brønsted-Lowry definitions while also catering to reactions where electron movement, rather than proton movement, is critical.
Examples & Analogies
Consider a handshake as an analogy for the Lewis theory. When two people meet, one person (the Lewis acid) reaches out for a handshake (accepts an electron pair), while the other (the Lewis base) offers their hand (donates an electron pair). This interaction forms a bond, just like the bond formed when BF₃ and NH₃ react.
Conjugate Acid-Base Pairs and Reaction Direction
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Conjugate Acid and Conjugate Base:
- Conjugate acids or bases are pairs formed after transfer of protons.
Strength Relationship:
- Strong acids have weak conjugate bases; weak acids have strong conjugate bases.
Detailed Explanation
Understanding conjugate acid-base pairs helps explain how acids and bases behave in reactions. When an acid donates a proton, it forms a conjugate base, while bases that accept a proton form conjugate acids. This naturally leads into the observation that strong acids have weaker conjugate bases and vice versa, which is vital for predicting reaction behavior and direction.
Examples & Analogies
Think of a relay race, where one runner is the acid passing the baton (proton) to the next runner (the base). The runner who just completed their leg (the acid) is now resting (the conjugate base), while the new runner (the conjugate acid) is taking off. The stronger and faster the runner (strong acid), the less likely they are to pass the baton effectively in future runs (resulting in a weaker conjugate base).
Key Concepts
-
Arrhenius Theory: Focuses on the production of H⁺ and OH⁻ ions in water.
-
Brønsted-Lowry Theory: Defines acids and bases in terms of proton donation and acceptance.
-
Lewis Theory: Defines acids and bases based on electron pair donation and acceptance.
-
Conjugate Acid-Base Pairs: Highlight the relationship between acids and their corresponding bases.
Examples & Applications
Hydrochloric acid (HCl) in water dissociates to produce H⁺ and Cl⁻.
Sodium hydroxide (NaOH) in water dissociates to produce Na⁺ and OH⁻.
Key Features:
Limited to aqueous solutions.
Explains acid-base behavior in terms of ions in water.
Limitations:
Cannot explain acid-base reactions in nonaqueous solvents.
Cannot classify substances like ammonia (NH₃) as a base.
Detailed Explanation: Arrhenius theory provides a specific definition of acids and bases based on their behavior in water. Arrhenius acids increase the concentration of H⁺ ions, while bases increase OH⁻ ions. However, its limitations include the inability to explain the behavior of these substances in non-solution contexts, such as reactions in solvents other than water.
Real-Life Example or Analogy: Think of Arrhenius acids and bases like a recipe for making lemonade. The lemon juice represents the acid, which contributes "sourness" (H⁺), and the sugar represents the "sweetness" (OH⁻). But this recipe only works when you have water (the solution); without it, you can't get lemonade, just like how these definitions only apply in an aqueous environment.
--
Chunk Title: Brønsted-Lowry Theory
Chunk Text: ### Definition:
A Brønsted-Lowry acid is a substance that donates a proton (H⁺) to another substance.
A Brønsted-Lowry base is a substance that accepts a proton (H⁺) from another substance.
Conjugate Acid-Base Pairs:
After donating a proton, an acid becomes its conjugate base.
After accepting a proton, a base becomes its conjugate acid.
Detailed Explanation: The Brønsted-Lowry theory enhances our understanding of acids and bases by focusing on proton transfer. This means that the definition of acids and bases is not restricted to water and can include various chemical reactions, allowing us to classify substances based on their proton donation or acceptance.
Real-Life Example or Analogy: Imagine two friends passing a basketball during a game. The friend throwing the ball represents the acid donating a proton, and the friend receiving it is the base that accepts the proton. After the pass, the thrower now stands without the ball (conjugate base), while the receiver becomes more involved in the game (conjugate acid).
--
Chunk Title: Lewis Theory
Chunk Text: ### Definition:
A Lewis acid is an electron-pair acceptor.
A Lewis base is an electron-pair donor.
Examples:
Borane (BF₃) and Ammonia (NH₃): BF₃ accepts an electron pair from NH₃, forming a coordinate bond.
Detailed Explanation: The Lewis theory shifts the focus from protons to electron pairs, enabling a clearer explanation for a wider range of reactions that do not involve protons directly. It encompasses both Arrhenius and Brønsted-Lowry definitions while also catering to reactions where electron movement, rather than proton movement, is critical.
Real-Life Example or Analogy: Consider a handshake as an analogy for the Lewis theory. When two people meet, one person (the Lewis acid) reaches out for a handshake (accepts an electron pair), while the other (the Lewis base) offers their hand (donates an electron pair). This interaction forms a bond, just like the bond formed when BF₃ and NH₃ react.
--
Chunk Title: Conjugate Acid-Base Pairs and Reaction Direction
Chunk Text: ### Conjugate Acid and Conjugate Base:
Conjugate acids or bases are pairs formed after transfer of protons.
Strength Relationship:
Strong acids have weak conjugate bases; weak acids have strong conjugate bases.
Detailed Explanation: Understanding conjugate acid-base pairs helps explain how acids and bases behave in reactions. When an acid donates a proton, it forms a conjugate base, while bases that accept a proton form conjugate acids. This naturally leads into the observation that strong acids have weaker conjugate bases and vice versa, which is vital for predicting reaction behavior and direction.
Real-Life Example or Analogy: Think of a relay race, where one runner is the acid passing the baton (proton) to the next runner (the base). The runner who just completed their leg (the acid) is now resting (the conjugate base), while the new runner (the conjugate acid) is taking off. The stronger and faster the runner (strong acid), the less likely they are to pass the baton effectively in future runs (resulting in a weaker conjugate base).
--
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Brønsted acids give H⁺ with cheer, bases accept, it’s all quite clear.
Stories
Imagine a party: Acids throw protons like confetti, while bases eagerly catch them. This interaction defines their roles!
Memory Tools
Arrhenius - A for aqueous; Brønsted - B for bond (proton transfer); Lewis - L for electrons (pair donation).
Acronyms
A-B-L
Arrhenius
Brønsted
Lewis - remember the progression of acid-base definitions.
Flash Cards
Glossary
- Arrhenius Acid
A substance that increases the concentration of hydrogen ions (H⁺) in aqueous solution.
- Arrhenius Base
A substance that increases the concentration of hydroxide ions (OH⁻) in aqueous solution.
- BrønstedLowry Acid
A substance that donates a proton (H⁺) to another substance.
- BrønstedLowry Base
A substance that accepts a proton (H⁺) from another substance.
- Conjugate AcidBase Pair
A pair of compounds that differ by the presence or absence of a proton.
- Lewis Acid
A species that accepts an electron pair.
- Lewis Base
A species that donates an electron pair.
Reference links
Supplementary resources to enhance your learning experience.
