Functional Groups
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Functional Groups
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome everyone! Today we're diving into functional groups. These are groups of atoms that give organic molecules their distinct chemical properties. Can anyone tell me why functional groups are important?

They determine how a molecule will react?

Exactly! They essentially dictate a compound's reactivity and polarity. Think of them as the 'identity' of a molecule that helps in predicting its chemical behavior.

What are some examples of functional groups?

Great question! We'll cover specific functional groups like alcohols, aldehydes, ketones, and more shortly. They represent a wide range of organic compounds.
Haloalkanes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s start with haloalkanes, which have the functional group —X where X is a halogen. Can anyone provide a general formula for haloalkanes?

Is it R-X?

Yes, that’s correct! The carbon-halogen bond is polar, which means there's some interesting reactivity there. What types of reactions do haloalkanes typically undergo?

They undergo nucleophilic substitutions, right?

Correct! The halogen can be replaced by a nucleophile in these reactions, making them quite important in synthetic organic chemistry.
Alcohols and Their Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next up are alcohols, characterized by the −OH group. What happens to their boiling points compared to alkanes?

I remember that alcohols have higher boiling points due to hydrogen bonding!

Exactly! That hydrogen bonding creates a significant impact on their solubility and boiling points. Can anyone name the types of alcohols based on their carbon bonds?

They can be primary, secondary, or tertiary.

Correct! Each type has different reactivity patterns as well, especially in oxidation reactions.
Reactivity of Aldehydes and Ketones
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s talk about aldehydes and ketones. Both contain the carbonyl group. What’s the main difference between them?

In aldehydes, the carbonyl carbon is bonded to at least one hydrogen, while in ketones, it’s bonded to two carbons.

Absolutely right! This structural difference leads to aldehydes being more reactive than ketones. Can anyone explain why this is significant?

Does it affect their oxidation potentials?

Yes! Aldehydes can be oxidized to carboxylic acids, whereas ketones are generally more resistant to oxidation. Good job!
Carboxylic Acids and Their Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s wrap up with carboxylic acids. They’ve got that −COOH group which is responsible for their acidic behavior. What makes them acidic?

They can donate an H+ ion, right?

Exactly! Their acidity comes from their ability to donate protons. What happens when they react with bases?

They form salts and water!

Correct! And just to summarize, understanding functional groups is essential for predicting chemical behavior in organic chemistry.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores the importance of functional groups in organic chemistry, detailing their structures, nomenclature, and the types of chemical reactions associated with each group, such as alcohols, aldehydes, ketones, and more.
Detailed
Functional Groups in Organic Chemistry
Functional groups are specific atoms or groups of atoms within organic molecules that are responsible for the characteristic chemical properties and reactions of those molecules. Each functional group can alter the properties of a compound significantly, affecting solubility, boiling point, and reactivity.
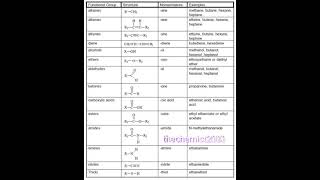
Below are various functional groups commonly encountered in organic chemistry:
- Haloalkanes (Alkyl Halides): Characterized by the presence of a halogen atom (F, Cl, Br, I) bonded to a carbon atom. Their general formula is R-X. The carbon-halogen bond is polar, making them susceptible to nucleophilic attack. Common reactions include nucleophilic substitution and elimination reactions.
- Alcohols: Featuring a hydroxyl group (−OH), resulting in their classification based on the number of carbon atoms directly bonded to the carbon linked to the hydroxyl (primary, secondary, tertiary). Alcohols show notable properties due to hydrogen bonding, such as higher boiling points compared to hydrocarbons. They can undergo oxidation, dehydration, and esterification reactions.
- Ethers: Defined by an oxygen atom bonded to two alkyl or aryl groups (−O−). They have the general formula R-O-R', and they are relatively unreactive and often good solvents for organic reactions. They do not exhibit hydrogen bonding.
- Aldehydes: Containing a carbonyl group (C=O) where the carbonyl carbon is bonded to at least one hydrogen atom. Aldehydes can undergo nucleophilic addition reactions and are easily oxidized to form carboxylic acids.
- Ketones: Similar to aldehydes, but the carbonyl carbon is bonded to two alkyl or aryl groups, making them less reactive. They are usually resistant to oxidation under mild conditions.
- Carboxylic Acids: Characterized by the presence of a carboxyl group (−COOH), they are weak acids that partially dissociate in water, forming strong hydrogen bonds and having high boiling points. They react with bases to form salts and can participate in esterification.
- Esters: Resulting from the reaction between alcohols and carboxylic acids, esters contain the −COO− group and often have distinctive smells, making them common in flavorings. They can undergo hydrolysis to revert to the acid and alcohol.
- Amines: Featuring nitrogen atoms bonded to carbon atoms, amines can be classified into primary, secondary, and tertiary based on the number of carbon groups attached to the nitrogen. They are basic and can form hydrogen bonds, affecting their boiling points.
- Amides: Containing a carbonyl group bonded to a nitrogen atom, amides are less reactive due to resonance stabilization. They are important in biological contexts, such as in peptide bonds between amino acids.
Overall, understanding functional groups enables chemists to predict the behavior of organic compounds and design molecules with desired properties and reactivity.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Functional Groups
Chapter 1 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Functional groups are specific atoms or groups of atoms within a molecule that are responsible for its characteristic chemical properties and reactions. They are often bonded to a hydrocarbon portion (represented as 'R' for an alkyl group or 'Ar' for an aryl group).
Detailed Explanation
Functional groups are important components of organic molecules. They define how the molecule behaves chemically. Think of them as the 'business end' of the molecule, determining its reactivity with other substances. Each functional group will have a specific structure and will interact differently during chemical reactions. The hydrocarbon part, represented as 'R' or 'Ar', forms the framework that the functional group is attached to, helping to define the overall molecule's behavior.
Examples & Analogies
Imagine functional groups like different tools in a toolbox. Each tool (functional group) has a specific purpose and can be used in particular ways. Just like how a hammer can drive nails and a screwdriver can turn screws, different functional groups will react in different ways with other molecules.
Haloalkanes (Alkyl Halides)
Chapter 2 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Functional Group: −X (where X is a halogen atom: F, Cl, Br, I).
● General Formula: R-X.
● Nomenclature: Named as a halogen substituent (prefix) on an alkane chain (e.g., chloroethane, 2-bromopropane, 1,2-dichloroethane).
● Properties:
○ Polarity: The carbon-halogen bond (C-X) is polar due to the electronegativity difference between carbon and the halogen, making the carbon slightly positive and susceptible to attack by nucleophiles.
○ Reactions: Primarily undergo nucleophilic substitution reactions (e.g., reaction with OH− to form alcohols, CN− to form nitriles, NH3 to form amines) where the halogen is replaced by a nucleophile. They can also undergo elimination reactions (in the presence of a strong base) to form alkenes.
○ Physical Properties: Boiling points generally increase with increasing molecular mass and with the size of the halogen atom. They are relatively insoluble in water.
Detailed Explanation
Haloalkanes are organic compounds that contain a halogen atom (F, Cl, Br, or I) bonded to a hydrocarbon. The bond between carbon and the halogen is polar, which means that the carbon atom acquires a slight positive charge, making it attractive to negatively charged species (nucleophiles). This allows haloalkanes to participate in reactions where the halogen can be replaced by other groups, such as hydroxyl (-OH) or amino (-NH2) groups. For example, when a haloalkane reacts with sodium hydroxide (NaOH), it can yield an alcohol by substituting the halogen atom. They also tend to have higher boiling points compared to similar alkane molecules because the larger halogen atoms increase the molecular mass.
Examples & Analogies
Consider a haloalkane like chloroethane as a piece of furniture with a removable part (the halo). If you wanted to replace that part with something else (like an alcohol), the furniture (haloalkane) will remain the same shape and function, but with a new feature that could give it different properties.
Alcohols
Chapter 3 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Functional Group: −OH (hydroxyl group).
● General Formula: R-OH.
● Nomenclature: Named by replacing the '-e' of the corresponding alkane with '-ol'. The position of the -OH group is indicated by a number (e.g., ethanol, propan-1-ol, propan-2-ol).
● Classification:
○ Primary (1°): The carbon atom bonded to the -OH group is attached to only one other carbon atom (e.g., ethanol).
○ Secondary (2°): The carbon atom bonded to the -OH group is attached to two other carbon atoms (e.g., propan-2-ol).
○ Tertiary (3°): The carbon atom bonded to the -OH group is attached to three other carbon atoms (e.g., 2-methylpropan-2-ol).
● Properties:
○ Hydrogen Bonding: The presence of the highly polar -OH group allows alcohols to form strong hydrogen bonds with each other and with water molecules. This significantly increases their boiling points compared to alkanes of similar molecular mass and makes smaller alcohols highly soluble in water.
Detailed Explanation
Alcohols are organic compounds containing a hydroxyl (-OH) group. The presence of this polar functional group enables alcohols to form hydrogen bonds, which greatly enhances their boiling points compared to alkanes. For instance, ethanol (an alcohol) has a boiling point of 78°C, while ethane (an alkane) is much lower at -89°C. The classification into primary, secondary, and tertiary alcohols allows chemists to predict how the alcohol might react based on the structure surrounding the -OH group. In brief, primary alcohols have one carbon atom connected to the carbon that bears the -OH group, while secondary alcohols have two, and tertiary have three.
Examples & Analogies
Think of alcohols like different types of family gatherings. A primary alcohol, like a small family dinner, involves just a few people (one carbon connected to the -OH). A secondary alcohol resembles a larger reunion where more relatives gather (two carbons), and a tertiary alcohol is like a big festival celebrating many family branches (three carbons). Just as more people means more interactions, more carbon atoms connected to -OH increase the complexity and reactivity of the alcohol.
Ethers
Chapter 4 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Functional Group: −O− (an oxygen atom bonded to two alkyl or aryl groups).
● General Formula: R-O-R′.
● Nomenclature: Typically named as alkoxyalkanes (e.g., CH3 OCH2 CH3 is methoxyethane). Common names involve naming the two alkyl groups alphabetically followed by 'ether' (e.g., CH3 OCH3 is dimethyl ether).
● Properties:
○ Lack of Hydrogen Bonding: Unlike alcohols, ethers do not have an -OH group and therefore cannot form hydrogen bonds with each other. This results in significantly lower boiling points than isomeric alcohols.
○ Solubility: Smaller ethers can form hydrogen bonds with water due to the oxygen's lone pairs, making them slightly soluble.
○ Reactivity: Generally unreactive, making them good solvents for many organic reactions.
Detailed Explanation
Ethers are characterized by an oxygen atom bonded to two alkyl or aryl groups. Unlike alcohols, ethers lack -OH groups, which means they cannot engage in hydrogen bonding with themselves, resulting in lower boiling points than similarly sized alcohols. For example, dimethyl ether is a gas at room temperature, while ethanol (an alcohol) is a liquid. Ethers can dissolve in water to some extent due to the presence of oxygen, which can interact with water molecules. Ethers are often used as solvents in chemical reactions due to their relatively inert nature, allowing other reactions to proceed without their interference.
Examples & Analogies
You can think of ethers like a bridge connecting two islands (the alkyl groups) but not having a roadway of its own (no hydrogen bonding). Without a direct road between them, the islands remain separate (lower boiling point). Just as a bridge makes it easier to reach both islands from a distance, ethers facilitate many chemical reactions as solvents without getting involved themselves.
Aldehydes
Chapter 5 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Functional Group: −CHO (a carbonyl group, C=O, where the carbonyl carbon is bonded to at least one hydrogen atom and one alkyl/aryl group).
● General Formula: R-CHO.
● Nomenclature: Named by replacing the '-e' of the corresponding alkane with '-al'. The aldehyde group is always at the end of a chain and its carbon atom is always numbered as 1 (e.g., methanal, ethanal, propanal).
● Properties:
○ Polarity: The carbonyl group (C=O) is polar, making aldehydes reactive.
○ Reactions: Undergo nucleophilic addition reactions (e.g., with HCN to form cyanohydrins, or with NaBH4 to form primary alcohols). They are easily oxidized to carboxylic acids.
○ Tests: Aldehydes are reducing agents. They give a positive result with:
■ Tollen's Reagent: A 'silver mirror' forms on the inside of the test tube.
■ Fehling's Solution / Benedict's Solution: A brick-red precipitate of Cu2 O forms.
Detailed Explanation
Aldehydes contain a carbonyl group that is attached to a hydrogen atom, giving them unique chemical properties. The carbonyl group is polar, which makes aldehydes reactive under various conditions, specifically suitable for nucleophilic addition reactions. They can be easily oxidized to form carboxylic acids, which provides insight into their reactivity. When testing for the presence of aldehydes, Tollen's reagent and Fehling's solution produce distinct visual changes, indicating the reducing nature of aldehydes. For example, Tollen's reagent creates a silver mirror, while Fehling's solution produces a colored precipitate.
Examples & Analogies
Aldehydes can be likened to actors on a stage. They have a dramatic presence (their reactivity) when they engage with other substances (nucleophiles), often leading to a transformation (oxidation to carboxylic acids). Similar to how a stage actor might change costumes, aldehydes can 'change' when they react with the appropriate reagents, showcasing their ability to shift forms and create something new.
Ketones
Chapter 6 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Functional Group: −CO− (a carbonyl group, C=O, where the carbonyl carbon is bonded to two alkyl or aryl groups).
● General Formula: R-CO-R′.
● Nomenclature: Named by replacing the '-e' of the corresponding alkane with '-one'. The position of the carbonyl group is indicated by a number (e.g., propanone, butan-2-one).
● Properties:
○ Polarity: Like aldehydes, the carbonyl group is polar.
○ Reactions: Undergo nucleophilic addition reactions (e.g., with HCN to form cyanohydrins, or with NaBH4 to form secondary alcohols).
○ Oxidation: Generally resistant to oxidation under mild conditions (stronger oxidizing agents are required, which typically cause C-C bond cleavage).
○ Tests: Do not react with Tollen's reagent or Fehling's solution.
Detailed Explanation
Ketones are similar to aldehydes concerning the presence of a carbonyl group; however, they differ structurally as the carbonyl carbon is bonded to two other carbon atoms, making them generally less reactive. The polarity of the carbonyl group allows for nucleophilic addition reactions, much like aldehydes. However, ketones are typically more resistant to oxidation. In practical applications, ketones do not give positive results with Tollen’s reagent or Fehling’s solution, which helps differentiate them from aldehydes.
Examples & Analogies
You can think of ketones as a well-organized library where all the books (carbon chains) are neatly aligned around a central table (the carbonyl group). While they are functional and can interact with visitors (nucleophiles) for information (reactions), they are less likely to change the state of the library (resist oxidation) compared to a more chaotic room (aldehyde) that is eager to undergo changes.
Carboxylic Acids
Chapter 7 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Functional Group: −COOH (a carboxyl group, containing both a carbonyl (C=O) and a hydroxyl (OH) group attached to the same carbon).
● General Formula: R-COOH.
● Nomenclature: Named by replacing the '-e' of the corresponding alkane with '-oic acid'. The carboxyl carbon is always numbered as 1 (e.g., methanoic acid, ethanoic acid).
● Properties:
○ Acidity: They are weak acids, meaning they partially dissociate in water to release H+ ions. The acidity is due to the resonance stabilization of the carboxylate anion (R-COO−). They react with bases to form salts.
○ Hydrogen Bonding: Form strong hydrogen bonds due to both the C=O and O-H groups. In solution, they often exist as dimers, leading to exceptionally high boiling points compared to alcohols of similar molecular mass.
○ Reactions:
■ Acid-Base Reactions: React with metals, metal oxides, hydroxides, and carbonates to form salts, H2 O, and CO2 (with carbonates).
■ Esterification: React with alcohols in the presence of an acid catalyst to form esters.
Detailed Explanation
Carboxylic acids are marked by the presence of a carboxyl group, which contributes to their acidic nature. They can donate a hydrogen ion (H+) when dissolved in water, making them weak acids. The resonance stabilization that occurs in carboxylate ions enhances their acidity. Additionally, the ability to form hydrogen bonds means carboxylic acids typically have higher boiling points than alcohols. They readily participate in reactions like esterification, where they combine with alcohols, highlighting their reactivity.
Examples & Analogies
Think of carboxylic acids like a restaurant that serves up 'food' (acids) but also facilitates takeout (reactions like esterification). When you bring in an alcohol (another food) under the right conditions, they can create a delicious dish (an ester), showcasing their versatility in the kitchen of organic chemistry.
Esters
Chapter 8 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Functional Group: −COO− (an ester linkage, with a carbonyl group attached to an oxygen which is then attached to another alkyl or aryl group).
● General Formula: R-COO-R′.
● Nomenclature: Named as alkyl alkanoates. The 'alkyl' part comes from the alcohol component (R'), and the 'alkanoate' part comes from the carboxylic acid component (R-COO-) (e.g., ethyl ethanoate from ethanol and ethanoic acid).
● Properties:
○ Smell: Often characterized by distinctive sweet or fruity smells, making them common in perfumes and food flavourings.
○ Hydrogen Bonding: Do not have an O-H group, so they cannot form hydrogen bonds with each other. This results in lower boiling points than carboxylic acids of similar molecular mass.
○ Reactions: Undergo hydrolysis (reaction with water) to revert to the parent carboxylic acid and alcohol. This can be acid-catalyzed or base-catalyzed (saponification).
Detailed Explanation
Esters are recognizable by their pleasant aromas and are formed from the reaction between a carboxylic acid and an alcohol. Their functional group features a carbonyl attached to an oxygen that is also linked to another carbon. This structure not only contributes to their sweet and fruity smells but also explains their relatively low boiling points when compared with carboxylic acids. Esters can undergo hydrolysis, allowing them to break down into their respective acid and alcohol.
Examples & Analogies
Consider esters as a sweet-smelling candy store (aromas). Without the typical connection (hydrogen bonds), the store remains less 'dense' than a carboxylic acid environment, leading to lower boiling points. If you were to pour a glass of water (hydrolyze) into the store, you could revert the candy back to its original ingredients (acid and alcohol), as if the sweet treats were sold out!
Amines
Chapter 9 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Functional Group: −NH2 (amino group), −NHR, or −NR2.
● General Formula: R-NH2 (primary amine).
● Nomenclature: Named by replacing the '-e' of the corresponding alkane with '-amine' (e.g., methylamine, ethylamine, propan-1-amine). Alternatively, they can be named as an amino- substituent.
● Classification:
○ Primary (1°): The nitrogen atom is bonded to one alkyl/aryl group and two hydrogen atoms (e.g., CH3 NH2).
○ Secondary (2°): The nitrogen atom is bonded to two alkyl/aryl groups and one hydrogen atom (e.g., CH3 NHCH3).
○ Tertiary (3°): The nitrogen atom is bonded to three alkyl/aryl groups (e.g., (CH3)3N).
● Properties:
○ Basicity: Amines are basic due to the lone pair of electrons on the nitrogen atom, which can accept a proton (H+). They react with acids to form salts.
○ Hydrogen Bonding: Primary and secondary amines can form hydrogen bonds with each other (N-H...N) and with water (N-H...O), leading to higher boiling points and solubility in water than alkanes of similar molecular mass. Tertiary amines cannot form hydrogen bonds with each other but can accept hydrogen bonds from water.
Detailed Explanation
Amines contain nitrogen and can function as basic compounds due to the presence of a lone electron pair on the nitrogen atom. They are classified based on how many carbon-containing groups they are connected to: primary (one carbon), secondary (two carbon), and tertiary (three carbon). Because of their ability to form hydrogen bonds, primary and secondary amines tend to have higher boiling points compared to alkanes of the same size. While tertiary amines don’t form hydrogen bonds with each other, they can still interact with water.
Examples & Analogies
Imagine amines as a family's home, with the nitrogen as the parent and the carbon groups as the children. Depending on how many children (carbon groups) live with the parent (nitrogen), the family structure (amine classification) can vary. When it comes to interactions with others (such as water), the family may be more or less involved depending on those connections, affecting how they respond to different environments (boiling points and reactivity).
Amides
Chapter 10 of 10
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Functional Group: −CONH2 (an amide group, containing a carbonyl group bonded directly to a nitrogen atom).
● General Formula: R-CONH2 (primary amide).
● Nomenclature: Named by replacing the '-oic acid' of the corresponding carboxylic acid with '-amide' (e.g., methanamide, ethanamide).
● Properties:
○ Hydrogen Bonding: Primary and secondary amides form strong hydrogen bonds (N-H...O=C and O=C...N-H), leading to exceptionally high boiling points. For example, ethanamide is a solid at room temperature.
○ Reactivity: Amides are relatively unreactive compared to other carbonyl compounds due to resonance stabilization of the amide linkage.
○ Reactions: Can undergo hydrolysis (cleavage by water) under strong acidic or basic conditions to yield a carboxylic acid and an amine (or ammonia). The peptide bonds that link amino acids in proteins are amide linkages.
Detailed Explanation
Amides have a functional group linking a carbonyl directly to a nitrogen atom, giving them unique properties. They can form strong hydrogen bonds due to the positions of their hydrogen and carbonyl atoms, which make their boiling points higher compared to similar compounds without such bonding. However, amides are generally less reactive compared to other carbonyl compounds because of resonance stabilization of the nitrogen-carbon bond. In biological systems, amides are important as they form peptide bonds that connect amino acids in proteins.
Examples & Analogies
Think of amides like a tightly knit family. They are strong and supportive (high boiling points due to hydrogen bonding) but also stable and less likely to change (lower reactivity). Just as family bonds (amide linkages) create a foundation for a larger community (proteins), these connections are vital for the structure and function of living organisms.
Key Concepts
-
Functional Groups: Specific atoms or groups of atoms that determine chemical properties.
-
Polarity in Bonds: Polar bonds, such as carbon-halogen, greatly influence reactivity.
-
Hydrogen Bonding: Present in alcohols and carboxylic acids, affecting boiling points and solubility.
-
Nucleophilic Substitution: Common reaction type for haloalkanes.
-
Acidity: Carboxylic acids are weak acids due to the presence of the carboxyl group.
-
Esters Formation: Created through reactions between alcohols and carboxylic acids.
Examples & Applications
Haloalkanes: Chloroethane (C2H5Cl) illustrates a simple alkyl halide.
Alcohols: Ethanol (C2H5OH) serves as both a common beverage and a solvent.
Aldehydes: Propanal (C3H6O) used in organic synthesis.
Ketones: Acetone (C3H6O) often used in nail polish remover.
Carboxylic Acids: Acetic acid (C2H4O2) found in vinegar.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Haloalkanes are such a show, with halogens in tow!
Stories
Imagine a village where everyone has a special task: the alcohols are the friendly hosts making great parties, while the aldehydes are the chefs cooking up delicious meals, and the ketones are the critics ensuring everything tastes just right.
Memory Tools
To remember functional groups: Have All Kids Eat All Colorful Eatables (Haloalkanes, Alcohols, Ketones, Esters, Carboxylic acids, Amines).
Acronyms
F.A.C.E.S (Functional groups, Alcohols, Carboxylic acids, Esters, Sulfonic acids) can help remember the main types.
Flash Cards
Glossary
- Functional Group
A specific group of atoms within a molecule that determines the molecule's chemical properties.
- Haloalkanes
Organic compounds where a halogen atom is bonded to an alkane carbon.
- Alcohols
Organic compounds containing one or more hydroxyl (−OH) groups.
- Aldehydes
Organic compounds containing a carbonyl group (C=O) with at least one hydrogen atom attached to the carbon.
- Ketones
Organic compounds containing a carbonyl group (C=O) bonded to two alkyl or aryl groups.
- Carboxylic Acids
Organic acids characterized by the presence of a carboxyl group (−COOH).
- Esters
Compounds formed from the reaction of an alcohol and a carboxylic acid.
- Amines
Organic compounds containing a nitrogen atom bonded to carbon atoms.
- Amides
Organic compounds containing a carbonyl group bonded to a nitrogen atom.
Reference links
Supplementary resources to enhance your learning experience.
