Isomerism (Structural, Geometric, Optical)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Isomerism
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will explore isomerism, which describes compounds having the same molecular formula but different arrangements of atoms. Can anyone tell me why this might be important in chemistry?

It may lead to different physical and chemical properties.

Exactly! These differences can affect reactivity, boiling points, and many other properties. Let's start with structural isomerism. Can anyone explain what that means?

Are they just different structures but the same formula?

Precisely! There are three types: chain isomerism, positional isomerism, and functional group isomerism. Let's dive into each one.
Structural Isomerism Types
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

First, we have chain isomerism. For example, butane and 2-methylpropane both have the formula C₄H₁₀. Butane has a straight chain, while 2-methylpropane is branched. What could be a property that differs between these two?

Maybe their boiling points?

Correct! The branching in 2-methylpropane lowers its boiling point compared to straight-chain butane. Now, can anyone explain positional isomerism?

It happens when the functional group is on different carbon atoms, right?

Exactly! For example, propan-1-ol and propan-2-ol have the same molecular formula but different hydroxyl group positions, and they behave differently chemically.
Stereoisomerism
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's talk about stereoisomerism, where molecules have the same connectivity but different spatial arrangements. What can you say about geometric isomerism?

It involves compounds with a double bond where you can't rotate the carbon bond easily, like in alkenes?

Yes! They can be 'cis' or 'trans'. For example, cis-but-2-ene has both methyl groups on the same side of the double bond, while trans-but-2-ene has them on opposite sides. Who can tell me a property affected by this difference?

The boiling point?

Exactly! Now let's discuss optical isomerism. What do you know about enantiomers?
Optical Isomerism
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Optical isomerism involves chiral centers—carbon atoms bonded to four different groups. Can anyone give an example of a compound that exhibits chirality?

Is lactic acid one?

Great example! Lactic acid has a chiral carbon and exists as two enantiomers. How do these enantiomers behave in plane-polarized light?

One rotates it clockwise and the other counter-clockwise.

Correct! This property is important in biological systems. Let's summarize what we’ve learned so far. Who can list the three types of isomerism we discussed today?
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores the various forms of isomerism, including structural isomerism—where molecules differ in connectivity—and stereoisomerism, which includes geometric and optical isomerism. Through examples, it illustrates how these variations impact the properties of compounds.
Detailed
Isomerism in Organic Chemistry
Isomerism in organic chemistry refers to the existence of compounds that share the same molecular formula yet differ in their structural or spatial arrangements. Such differences can lead to significant variations in the compounds' properties and reactivity.
1. Structural (Constitutional) Isomerism
Structural isomers differ in the connectivity of their atoms. There are three main types:
- Chain Isomerism: Compounds have different carbon chain arrangements (e.g., butane vs. 2-methylpropane, both C₄H₁₀).
- Positional Isomerism: Same carbon skeleton and functional group, but in different positions (e.g., propan-1-ol vs. propan-2-ol, both C₃H₈O).
- Functional Group Isomerism: Identical molecular formulas but different functional groups (e.g., ethanol vs. methoxymethane, both C₂H₆O).
2. Stereoisomerism
Stereoisomers share the same connectivity but differ in spatial arrangement, dividing into two main categories:
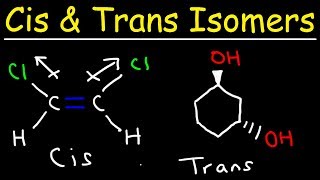
- Geometric Isomerism (Cis-Trans Isomerism): Arises from restricted rotation around a bond, common in alkenes and cyclic compounds. Isomers can be 'cis' (same side) or 'trans' (opposite sides), affecting properties like boiling points.
- Optical Isomerism (Enantiomerism): Involves mirror-image isomers (enantiomers) that cannot be superimposed. These typically exist due to chiral centers (e.g., lactic acid). Enantiomers rotate polarized light in opposite directions, leading to distinct optical activities.
Understanding isomerism is crucial in organic chemistry as it affects the behavior and reactivity of compounds, significantly impacting fields such as pharmaceuticals and biochemistry.
Youtube Videos
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Isomerism
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Isomers are molecules that share the same molecular formula but possess different arrangements of atoms. This difference in arrangement results in distinct physical and chemical properties.
Detailed Explanation
Isomerism refers to the phenomenon where two or more compounds have the same molecular formula (meaning they contain the same number and types of atoms) but differ in how those atoms are arranged or connected. Because their structures differ, isomers often exhibit different physical and chemical properties. This concept is essential in organic chemistry because it highlights how subtle changes in molecular structure can lead to significant differences in behavior and reactivity.
Examples & Analogies
Think of isomers like different arrangements of the same building blocks. Imagine you have a set of Lego blocks. You can build a house in one design (one arrangement) or a car in another design using the same blocks. Even though both creations use the same blocks, they function very differently, just as isomers do.
Structural (Constitutional) Isomerism
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Structural (Constitutional) Isomerism: Structural isomers have the same molecular formula but differ in the sequence in which their atoms are connected (their connectivity).
- Chain Isomerism: Occurs when compounds with the same molecular formula have different arrangements of the carbon backbone. This means the carbon chain can be straight or branched.
- Positional Isomerism: Occurs when compounds with the same molecular formula have the same carbon skeleton and the same functional group, but the functional group (or a substituent) is located at a different position on the carbon chain.
- Functional Group Isomerism: Occurs when compounds with the same molecular formula possess different functional groups.
Detailed Explanation
Structural isomerism is a type of isomerism where isomers differ in how their atoms are connected. It can be categorized into three types:
- Chain Isomerism: This occurs when the carbon skeleton differs, leading to straight-chain versus branched structures. For example, butane (straight chain) and 2-methylpropane (branched) have different physical properties despite having the same molecular formula (C₄H₁₀).
- Positional Isomerism: In this case, isomers have the same carbon structure and functional group, but the location of the functional group varies. For instance, propan-1-ol and propan-2-ol both contain the same atoms but differ in the position of the hydroxyl group.
- Functional Group Isomerism: This happens when compounds with the same molecular formula contain different functional groups. A classic example is ethanol (an alcohol) and methoxymethane (an ether), both with the formula C₂H₆O but vastly different chemical reactivities.
Examples & Analogies
Imagine the difference between a pencil and a pen. They may share similar components (same types of materials and a similar overall function of writing) but have different structures and designs which give them distinct features and functionalities. This is akin to how structural isomers share the same molecular formula but have different connectivity and properties.
Stereoisomerism
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Stereoisomerism: Stereoisomers have the same molecular formula and the same connectivity of atoms, but they differ in the three-dimensional spatial arrangement of their atoms.
- Geometric Isomerism (cis-trans Isomerism): This type of isomerism arises from restricted rotation around a bond. The most common cases involve compounds with a carbon-carbon double bond (C=C) or cyclic structures.
- Optical Isomerism (Enantiomerism): This type of isomerism occurs in molecules that are non-superimposable mirror images of each other.
Detailed Explanation
Stereoisomerism involves isomers that have the same molecular formula and atomic connectivity but differ in how their atoms are arranged in three-dimensional space. There are two key types:
- Geometric Isomerism (cis-trans): This occurs mainly around double bonds or in cyclic compounds where rotation is restricted. For alkenes, if identical or priority groups are on the same side of the double bond, it is a cis isomer; if they are on opposite sides, it's a trans isomer. An example can be drawn from but-2-ene which can exist as cis-but-2-ene and trans-but-2-ene.
- Optical Isomerism: This arises when molecules have chiral centers—carbon atoms bonded to four different substituents, making them non-superimposable mirror images known as enantiomers. These isomers have identical physical properties but differ in how they interact with polarized light, showing optical activity.
Examples & Analogies
Think of chiral molecules like a pair of gloves – you cannot wear a left glove on your right hand or vice versa, even though they are made of the same material. Similarly, optical isomers may have the same atoms but cannot be superimposed onto each other, leading to unique behaviors in chemical interactions.
Geometric Isomerism
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Geometric Isomerism (cis-trans Isomerism): This type of isomerism arises from restricted rotation around a bond. The most common cases involve compounds with a carbon-carbon double bond (C=C) or cyclic structures. Conditions for Geometric Isomerism in Alkenes: Each carbon atom of the double bond must be bonded to two different groups.
Detailed Explanation
Geometric isomerism specifically addresses the orientation of groups around a double bond or within cyclic structures. Because double bonds cannot rotate freely, the positioning of attached groups relative to the double bond becomes crucial.
To exhibit geometric isomerism, both carbon atoms involved in the double bond must have two different substituents. If they do, we can classify the isomers as cis (same side of the double bond) or trans (opposite sides). This leads to distinct physical properties, including differing boiling and melting points, due to variations in molecular polarity and how tightly molecules can pack together.
Examples & Analogies
Imagine a seesaw on a playground. If you and your friend sit on opposite ends, it balances well (trans). But if both sit on the same side, it tilts significantly (cis). This difference often results in how the seesaw operates, just as the spatial arrangement in geometric isomers affects their stability and physical properties.
Optical Isomerism
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Optical Isomerism (Enantiomerism): This type of isomerism occurs in molecules that are non-superimposable mirror images of each other. Conditions for Optical Isomerism: The presence of a chiral center (or stereocenter). A chiral carbon atom is a carbon atom bonded to four different groups.
Detailed Explanation
Optical isomerism occurs when molecules are mirror images of one another, like your left and right hands, yet cannot be overlapped. This phenomenon occurs due to the presence of chiral centers in the molecules, where a carbon atom is bonded to four distinct groups.
These non-superimposable mirror images are termed enantiomers and exhibit identical physical properties except in how they interact with plane-polarized light. One enantiomer will rotate the light clockwise while the other does so counterclockwise, leading to the terms 'dextrorotatory' and 'levorotatory' respectively.
Examples & Analogies
Think of a pair of scissors: if you hold one side, it looks like a mirror image of the other but they can't be put on top of each other and align perfectly. Like chiral compounds, they can behave differently even though they're essentially made of the same components.
Key Concepts
-
Isomerism: Phenomenon where compounds have the same molecular formula but different arrangements.
-
Structural Isomerism: Types include chain, positional, and functional group isomerism.
-
Geometric Isomerism: Arises from restricted rotation leading to cis and trans configurations.
-
Optical Isomerism: Involves enantiomers that are non-superimposable mirror images due to chiral centers.
Examples & Applications
Butane and 2-methylpropane (C₄H₁₀) exhibit chain isomerism.
Propan-1-ol and propan-2-ol (C₃H₈O) demonstrate positional isomerism.
Ethanol (alcohol) and methoxymethane (ether) show functional group isomerism.
Cis- and trans-but-2-ene illustrate geometric isomerism.
Lactic acid has two optical isomers due to its chiral carbon.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Isomers play a game, same formula, but not the same name.
Stories
Imagine twins, Bob and Rob, identical in looks (their formula), but they dress completely differently (their structures).
Memory Tools
C for Chain, P for Position, F for Functional – remember these isomer types!
Acronyms
S.G.-O
Structural
Geometric
Optical – the three kinds of isomers to know.
Flash Cards
Glossary
- Isomerism
The occurrence of compounds with the same molecular formula but different arrangements of atoms.
- Structural Isomerism
Isomers that differ in the sequence of connections among their atoms.
- Geometric Isomerism
Stereoisomers that arise from restrictions in rotation around a bond, leading to different spatial arrangements.
- Optical Isomerism
Isomers that are non-superimposable mirror images of each other, often involving chiral centers.
Reference links
Supplementary resources to enhance your learning experience.
