Visualizing Bonds and Shapes: Lewis Structures and VSEPR Theory
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Lewis Structures
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're diving into Lewis structures, which are essential for visualizing how atoms bond in a molecule. Can someone explain what a Lewis structure is?

It's a diagram that shows the valence electrons for atoms in a molecule!

Exactly! Lewis structures help us comprehend how electrons are shared. To draw one, we follow a series of steps. Who can name one of these steps?

Counting the total number of valence electrons?

Great job! We start by counting the total valence electrons for all atoms. This is our first step and the key to representing the bonds accurately.

What comes after that?

Next, we determine the central atom, usually the least electronegative. Remember, hydrogen can never be central! Let's recap: total valence electrons, choose the central atom... what’s next?

We draw single bonds to connect the central atom to the outer atoms!

Perfect! Once we've drawn those single bonds, we distribute the remaining electrons to complete the octets around the outer atoms. Remember: the goal is to achieve stable configurations!

Lastly, if the central atom still doesn’t have an octet, we can form double or triple bonds. So, who can summarize the steps we discussed?

Count valence electrons, find the central atom, draw bonds, complete outer octets, and form multiple bonds as needed!

Exactly! These steps are key to visualizing molecular structures with Lewis diagrams.
Understanding VSEPR Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we have our Lewis structures, let’s learn about VSEPR theory! Who knows what VSEPR stands for?

Valence Shell Electron Pair Repulsion!

Exactly! VSEPR theory says that electron pairs will arrange themselves as far apart as possible to minimize repulsion. What are some examples of electron domains?

Single bonds, double bonds, and lone pairs!

Right again! Each of those counts as one electron domain. Can anyone tell me how we can categorize these domains into shapes?

Well, if there are two electron domains, the shape is linear!

Correct! It gives us a bond angle of 180 degrees. What about three electron domains?

That would be trigonal planar, with 120-degree angles!

Excellent! And remember, shapes can be influenced by lone pairs. What happens when a lone pair is present?

The bond angles can change because lone pairs push the bonding pairs closer together!

That's exactly right! Understanding how lone pairs affect geometry is crucial for predicting shapes.
Applying VSEPR to Determine Molecular Geometry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s apply what we've learned with some examples. Can someone give me a molecule with four electron domains?

Methane, CH4!

Correct! What’s its geometry?

It's tetrahedral with an ideal bond angle of 109.5 degrees!

Well done! Let's examine ammonia, NH3. How many lone pairs does it have?

One lone pair.

Great! Given that, what would its molecular shape be, and how does the lone pair affect the angles?

It’s trigonal pyramidal and has bond angles less than 109.5 degrees because the lone pair pushes the bonds closer.

Exactly! Now, what about water, H2O?

Water has two lone pairs and two bonds, making it bent with bond angles of around 104.5 degrees.

Perfect! All these examples show how VSEPR can effectively predict molecular shapes based on electron domains and lone pairs!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides an overview of Lewis structures to represent electron arrangements and introduces VSEPR theory to predict molecular geometry. Key concepts such as electron domains, bond formation, and the roles of lone pairs in determining shapes are emphasized.
Detailed
In this section, we explore Lewis structures as essential tools for illustrating the arrangement of valence electrons in molecules or polyatomic ions. These diagrams provide insights into how atoms bond through shared electrons and where lone pairs are located. To effectively draw Lewis structures, one must follow several steps: counting total valence electrons, identifying the central atom, establishing single bonds, completing outer atom octets, placing remaining electrons, and forming multiple bonds if needed. Following the visualization of bonds, VSEPR (Valence Shell Electron Pair Repulsion) theory enables us to predict the three-dimensional shapes of molecules by analyzing electron domains surrounding a central atom. These domains can represent bonds (single, double, triple) and lone pairs, which dictate both electron domain geometry and molecular geometry. Understanding geometries including linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral configurations, along with adjustments for lone pairs, is crucial for grasping molecular interactions and properties.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Lewis Structures
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Understanding the arrangement of valence electrons in atoms is the first step to predicting how they will bond.
Lewis Structures (Electron Dot Structures): Lewis structures are simple, yet powerful, diagrams that represent the valence electrons of atoms within a molecule or polyatomic ion. They provide a visual framework for understanding how electrons are shared in covalent bonds and where non-bonding (lone) pairs of electrons are located. The goal in drawing Lewis structures is generally to achieve stable electron configurations, typically an octet (eight valence electrons) for most atoms, and a duet (two valence electrons) for hydrogen.
Detailed Explanation
Lewis structures are diagrams used to visualize the arrangement of valence electrons in a molecule. Valence electrons are the outermost electrons of an atom that play a key role in chemical bonding. By drawing a Lewis structure, we can easily see how atoms are bonded and where lone pairs of electrons are, which helps determine the molecule's shape and properties. The overall goal when creating these structures is to ensure that atoms achieve stable electron configurations: most atoms aim for an octet (eight electrons), while hydrogen aims for two (a duet).
Examples & Analogies
Think of Lewis structures like a seating chart for a group of friends at a dinner party. Just as you want to arrange your friends in a way that they are comfortable and enjoy each other's company (stability), Lewis structures help place electrons (friends) around atoms (people) to achieve the most stable arrangement.
Steps to Draw Lewis Structures
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Steps to Draw Lewis Structures:
1. Count the total number of valence electrons: Sum the valence electrons for all atoms in the molecule or ion. For polyatomic ions, add one electron for each negative charge and subtract one electron for each positive charge.
2. Determine the central atom: This is usually the least electronegative atom, as it is most likely to share electrons with multiple other atoms. Hydrogen can never be a central atom as it only forms one bond.
3. Draw single bonds: Connect the central atom to all the outer (terminal) atoms with a single covalent bond. Each single bond uses two valence electrons.
4. Complete octets on outer atoms: Distribute the remaining valence electrons as lone pairs on the outer atoms until each outer atom (except hydrogen, which needs only a duet) has an octet.
5. Place remaining electrons on the central atom: If any valence electrons are still unaccounted for, place them as lone pairs on the central atom.
6. Form multiple bonds if necessary: If, after placing all electrons, the central atom does not have an octet, convert one or more lone pairs from an adjacent outer atom into double or triple bonds between the central and outer atoms. This increases the number of shared electrons to satisfy the central atom's octet.
Detailed Explanation
Drawing Lewis structures involves a systematic approach:
1. Count valence electrons: Calculate the total number of valence electrons from all participating atoms. Remember to adjust for charge if working with ions.
2. Identify the central atom: Generally, the atom with the lowest electronegativity is chosen as the central atom since it can bond with more atoms. Hydrogen can’t be central.
3. Create single bonds: Connect the central atom to outer atoms using single bonds—this uses up some valence electrons.
4. Octet completion for outer atoms: Distribute any remaining electrons as lone pairs until outer atoms reach stable configurations.
5. Lone pairs on central atom: If any electrons remain, place them as lone pairs on the central atom.
6. Multiple bonds: If the central atom still doesn't have an octet, adjust by forming double or triple bonds using lone pairs from surrounding atoms. This ensures the central atom achieves stability.
Examples & Analogies
Imagine you are organizing a group project where everyone needs to contribute (representing bonds). First, you count how many team members (valence electrons) you have. You then decide who leads the project (central atom), connect individuals (single bonds), and make sure everyone has a direct role (complete octets). If someone is left out and needs to contribute more (multiple bonds), you adjust the roles to ensure everyone is included and satisfied.
VSEPR Theory Overview
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
VSEPR Theory (Valence Shell Electron Pair Repulsion Theory): Once a Lewis structure is drawn, VSEPR theory provides a straightforward method for predicting the three-dimensional geometry (shape) of molecules and polyatomic ions. The fundamental principle of VSEPR theory is that electron domains around a central atom will arrange themselves as far apart as possible in three-dimensional space to minimize repulsion between them.
Detailed Explanation
VSEPR theory explains how the geometry of a molecule can be predicted based on the arrangement of electron pairs around the central atom. It states that because electrons are negatively charged, they repel each other and will try to spread out as much as possible to minimize this repulsion. This results in specific shapes for molecules, such as linear, trigonal planar, or tetrahedral, depending on how many electron domains are around the central atom: these can either be bonding pairs (bonds to other atoms) or lone pairs (non-bonding electrons).
Examples & Analogies
Think of the VSEPR theory like arranging chairs in a room. If you have a few friends (electron domains) and want everyone to comfortably sit (minimize repulsion), they will spread out to find the best spots, avoiding sitting too close to each other. This natural spreading out results in a layout where chairs (the shape of the molecule) have specific arrangements depending on how many friends are in the room.
Common Electron Domain Geometries
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Common Electron Domain Geometries (and corresponding Molecular Geometries when no lone pairs are present):
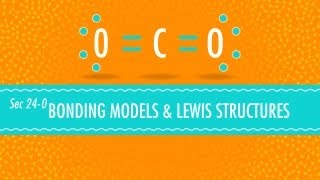
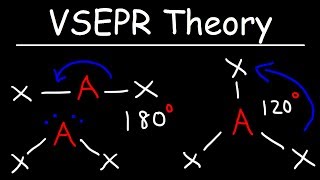
- 2 Electron Domains: The electron domains arrange linearly, resulting in a linear molecular geometry (e.g., carbon dioxide, CO2; beryllium chloride, BeCl2). Bond angle is 180°.
- 3 Electron Domains: The electron domains arrange in a trigonal planar fashion, leading to a trigonal planar molecular geometry (e.g., boron trifluoride, BF3; sulfur trioxide, SO3). Bond angle is 120°.
- 4 Electron Domains: The electron domains arrange tetrahedrally, giving a tetrahedral molecular geometry (e.g., methane, CH4; silicon tetrachloride, SiCl4). Ideal bond angle is 109.5°.
- 5 Electron Domains (HL): The electron domains arrange in a trigonal bipyramidal pattern, resulting in a trigonal bipyramidal molecular geometry (e.g., phosphorus pentachloride, PCl5). This geometry has two distinct positions: axial and equatorial, with bond angles of 90° and 120°.
- 6 Electron Domains (HL): The electron domains arrange octahedrally, leading to an octahedral molecular geometry (e.g., sulfur hexafluoride, SF6). Bond angles are 90°.
Detailed Explanation
Knowing the number of electron domains is essential for predicting the geometry of a molecule. Here's how these geometries work:
- 2 Electron Domains lead to a linear shape with a bond angle of 180°, like in carbon dioxide (CO2).
- 3 Electron Domains create a trigonal planar shape with bond angles of 120°, as seen in boron trifluoride (BF3).
- 4 Electron Domains result in a tetrahedral shape with bond angles of about 109.5° (e.g., methane, CH4).
- 5 Electron Domains form a trigonal bipyramidal shape, which includes both axial and equatorial positions, often appearing in phosphorus pentachloride (PCl5).
- 6 Electron Domains result in an octahedral shape with 90° bond angles, seen in sulfur hexafluoride (SF6). Each of these arrangements minimizes electron repulsion and helps determine bond angles and molecular shape.
Examples & Analogies
Consider laying out your garden (the molecule). If you have two flower beds (electron domains), you'll place them straight across from each other (linear). If three beds are included, you'd arrange them in a flat triangle (trigonal planar). Four beds would create a more pyramid-like structure (tetrahedral). With five or six beds, your layout gets complex, needing careful spacing to ensure each bed has room to grow (trigonal bipyramidal or octahedral shapes).
Lone Pairs and Molecular Geometry
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Predicting Molecular Geometry with Lone Pairs: Lone pairs of electrons occupy more space around the central atom than bonding pairs because their electron density is concentrated closer to the nucleus of the central atom and is not shared between two nuclei. Consequently, lone pairs exert stronger repulsive forces on other electron domains. This increased repulsion distorts the ideal bond angles predicted by the electron domain geometry, leading to distinct molecular geometries.
Examples with 4 electron domains (illustrating lone pair effects):
- Methane (CH4): The central carbon atom has 4 bonding pairs and 0 lone pairs. Both electron domain and molecular geometry are tetrahedral with ideal bond angles of 109.5°.
- Ammonia (NH3): The central nitrogen atom has 3 bonding pairs and 1 lone pair. The electron domain geometry is tetrahedral, but the lone pair's greater repulsion pushes the three N-H bonding pairs closer together, resulting in a trigonal pyramidal molecular geometry with bond angles of approximately 107°.
- Water (H2O): The central oxygen atom has 2 bonding pairs and 2 lone pairs. The electron domain geometry is tetrahedral. The two lone pairs exert even stronger repulsive forces, pushing the two O-H bonding pairs even closer, leading to a bent or V-shaped molecular geometry with bond angles of approximately 104.5°.
Detailed Explanation
When predicting molecular geometry, it's crucial to consider the effect of lone pairs of electrons. These lone pairs take up more space and create more repulsion than bonding pairs because they are located only near the nucleus without being shared with another atom. As a result, they distort the ideal bond angles. For instance:
- Methane (CH4) has no lone pairs, so it maintains the tetrahedral shape with 109.5° angles.
- Ammonia (NH3) has one lone pair, causing slight distortion and resulting in a trigonal pyramidal shape with bond angles around 107°.
- Water (H2O) has two lone pairs, causing significant repulsion that leads to a bent shape with bond angles of about 104.5°.
Examples & Analogies
Imagine a game of musical chairs for a group of people. In a perfect game with no disruptions (like lone pairs), each person can sit in their assigned chair (ideal angles). However, when one person sits out for a moment (lone pair), it creates a bit of chaos, causing those left to huddle closer together (distorted angles). The more people out, the more crowded it feels, similar to how lone pairs affect the spacing between bonding pairs.
Molecular Polarity
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Molecular Polarity: The overall polarity of a molecule is a critical property that influences its physical behavior, such as solubility and boiling point. Molecular polarity depends on two interconnected factors:
1. The polarity of individual bonds: Determined by the electronegativity difference between the bonded atoms. Polar bonds create individual bond dipoles.
2. The molecular geometry: The arrangement of these individual bond dipoles in three-dimensional space determines whether they cancel each other out or add up to create an overall molecular dipole moment. If a molecule is perfectly symmetrical and the bond dipoles cancel each other out, the molecule is non-polar (e.g., carbon tetrachloride (CCl4) and carbon dioxide (CO2)). If the bond dipoles do not cancel out due to an asymmetrical arrangement, the molecule is polar (e.g., water (H2O) and ammonia (NH3)).
Detailed Explanation
Molecular polarity refers to the distribution of electrical charges within a molecule. It plays a vital role in physical behaviors such as how molecules interact with each other or dissolve in solvents. Two main factors determine polarity:
1. Polarity of individual bonds: Bonds between atoms can be polar if there is a significant electronegativity difference, resulting in dipoles where electrons are unevenly distributed.
2. Molecular geometry: The shape of the molecule determines whether these dipoles cancel each other out (making the molecule non-polar) or add up (making it polar). For example, carbon dioxide (CO2) is symmetrical and non-polar, while water (H2O) has an asymmetrical shape, making it polar.
Examples & Analogies
Consider a seesaw in a playground. If two kids of equal weight sit on opposite sides (polar bonds canceling out), it stays level (non-polar). But if one side had a heavier kid (asymmetrical arrangement), the seesaw tips to that side (polar), representing how molecules behave depending on their shape and bond distribution.
Key Concepts
-
Lewis Structures: Visual representation of valence electrons and bonds.
-
VSEPR Theory: Predicts molecular shape based on electron domains.
-
Electron Domains: Regions of high electron density, including bonds and lone pairs.
-
Central Atom: The atom bonded to multiple atoms, key to structure.
-
Bond Angles: Angles formed between bonded atoms, affecting molecule shape.
Examples & Applications
Water (H2O) is an example of a bent molecule due to two lone pairs on oxygen and two hydrogen bonds.
Carbon dioxide (CO2) is linear because it has no lone pairs on the central carbon atom.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Lewis structures help to show, how atoms together do grow.
Stories
Imagine electrons at a party, spreading out to avoid being too close; they choose different corners of the room, creating the perfect molecular shape!
Memory Tools
C-Count, S-Select, B-Bond, O-Octet, R-Redistribute: Remember CSBOR for drawing Lewis Structures.
Acronyms
VSEPR
Valence Shell Electron Pair Repulsion - remember this for molecular shapes.
Flash Cards
Glossary
- Lewis Structure
Diagrams that represent the valence electrons of atoms within a molecule, showing how they are bonded.
- VSEPR Theory
A theory used to predict the three-dimensional geometry of molecules based on electron pair repulsion.
- Electron Domain
Any region of high electron density surrounding a central atom, including single bonds, double bonds, and lone pairs.
- Central Atom
The atom in a Lewis structure that is bonded to multiple other atoms, usually the least electronegative.
- Bond Angle
The angle formed between three atoms in a molecule, dictated by the spatial arrangement of electron domains.
Reference links
Supplementary resources to enhance your learning experience.
