Gas Laws
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Ideal Gas Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we are going to talk about the Ideal Gas Law. Who can tell me what it relates?

It relates pressure, volume, temperature, and moles of gas.

Exactly! The equation is PV = nRT. Can anyone tell me what each variable stands for?

P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature.

Great job! Remembering these components can be simplified with the acronym 'PVnRT'. Now, how do changes in each variable affect the others?

If you increase the volume while keeping moles and temperature constant, pressure decreases, right?

That's correct! This is known as Boyle's Law, which is a part of the Ideal Gas Law. Let's summarize: the Ideal Gas Law connects multiple gas behaviors into one versatile equation.
Kinetic Theory of Gases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s dive into the Kinetic Theory of Gases. Can anyone explain how this theory describes gases?

It says that gases are made of many small particles in constant, random motion.

Correct! This leads to the idea that the volume of gas particles is negligible compared to the container's volume. What else do we know about interactions between these particles?

There are no intermolecular forces between the particles.

Exactly! And what about their collisions?

The collisions are perfectly elastic, which means they don’t lose kinetic energy.

Great points! The average kinetic energy of the gas is also proportional to the temperature. Higher temperatures mean higher energy!

So if we heat a gas, it moves faster!

That's right! Let’s summarize: the Kinetic Theory informs us how we understand gas behavior at a molecular level, reinforcing our understanding of the Ideal Gas Law.
Pressure and Molecular Motion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about how pressure arises in gases. What causes the pressure inside a balloon, for instance?

It's from the collisions of gas molecules with the walls of the balloon.

Correct! The more frequent these collisions, the higher the pressure. Can anyone relate this to volume or temperature changes?

If we decrease the volume of the balloon, the molecules will collide more often, increasing the pressure.

Exactly, that's Boyle's Law in action! Let’s recap: pressure in a gas results from molecular collisions, and adjusting volume or temperature can significantly affect it.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Gas Laws, including the Ideal Gas Law and principles from the Kinetic Theory of Gases, highlight how gases behave under different conditions related to pressure, volume, and temperature. Key concepts include the relationships defined by the Ideal Gas Law and the impact of molecular motion on gas behavior.
Detailed
Gas Laws
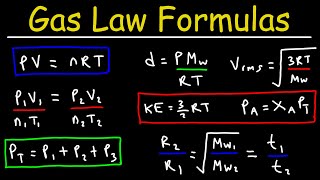
Gas Laws encompass the principles that govern the behavior of gases in different states and conditions. The Ideal Gas Law relates pressure (P), volume (V), temperature (T), and the number of moles (n) of a gas through the equation PV = nRT where R is the universal gas constant. This law is significant as it integrates previously known gas laws, including Boyle's law and Charles's law.
The Kinetic Theory of Gases offers a microscopic perspective, explaining that gases consist of numerous particles in perpetual and random motion. In this framework, the volume occupied by gas particles is negligible compared to the volume of the container, and there are no significant intermolecular forces amongst them. Collisions between particles and with container walls are considered perfectly elastic, meaning there is no energy loss in these collisions. The average kinetic energy of gas molecules correlates directly with the absolute temperature.
Furthermore, gas pressure arises from these particle collisions with container walls; more frequent and forceful collisions mean higher pressure. Understanding these fundamental laws integrates important concepts of physical chemistry and thermodynamics part of the broader study of the particulate nature of matter.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Ideal Gas Law
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ideal gas law relates the pressure, volume, temperature, and amount of an ideal gas:
PV=nRT
Where:
● P: Pressure (Pa)
● V: Volume (m³)
● n: Number of moles
● R: Universal gas constant (8.314 J/mol·K)
● T: Temperature (K)
Detailed Explanation
The ideal gas law is a key equation in understanding how gases behave. It relates five variables: pressure (P), volume (V), temperature (T), and the amount of gas in moles (n). The universal gas constant (R) connects these variables with numerical relationships. Each term in the equation has a specific unit, and when you know three of these values, you can calculate the fourth. This law applies mainly to ideal gases, theoretical gases that perfectly follow this equation at all conditions.
Examples & Analogies
Think of a flexible balloon filled with air. If you heat it (raising the temperature T), the air molecules inside move faster, causing the pressure (P) to increase if the volume (V) stays the same. This example illustrates how the ideal gas law helps us understand real-world scenarios involving gases.
Kinetic Theory of Gases
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The kinetic theory provides a microscopic explanation of gas behavior:
● Gases consist of a large number of small particles (atoms or molecules) in constant, random motion.
● The volume of the gas particles is negligible compared to the volume of the container.
● There are no intermolecular forces between the gas particles.
● Collisions between gas particles and with the walls of the container are perfectly elastic.
● The average kinetic energy of the gas particles is proportional to the absolute temperature of the gas.
Detailed Explanation
The kinetic theory of gases explains how gases behave on a microscopic level. It tells us that gas is made up of tiny particles that are constantly moving and colliding with each other and the walls of their container. The fact that their volume is negligible means we can consider the gases to occupy the entire container space without worrying about their size. The theory also states that these collisions are elastic, meaning no energy is lost in the form of heat during collisions. The temperature of the gas reflects the average energy of these particles – the higher the temperature, the faster the particles move.
Examples & Analogies
Imagine a crowded room where people are constantly bumping into each other while moving around. Even though everyone is in constant motion, none of them stick to each other; they bounce off, just like gas molecules do. The faster they move (like increasing the room's temperature), the more often they collide with each other and with the walls.
Pressure and Molecular Motion
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Pressure in a gas arises from collisions of gas particles with the walls of the container. The more frequent and forceful the collisions, the higher the pressure.
Detailed Explanation
The pressure exerted by a gas is directly related to how often and how forcefully its particles collide with the walls of their container. If there are more particles or they are moving faster (higher temperature), collisions happen more frequently, leading to increased pressure. This concept helps explain why inflating a tire increases pressure or why a sealed container can burst if heated.
Examples & Analogies
Consider a soda can. When you shake it, the gas inside moves rapidly and collides with the can's walls more frequently, increasing the pressure. When you open it, the pressure is released suddenly, and the gas escapes rapidly, often causing a fizzy eruption!
Key Concepts
-
Ideal Gas Law: Describes the relationship between pressure, volume, temperature, and amount of gas.
-
Kinetic Theory: Explains gas behavior based on particle motion and interactions.
-
Pressure: Resulting force from gas particle collisions within a container.
Examples & Applications
When a bicycle pump is used, the volume of the air inside decreases, increasing the pressure, which forces air into the tire.
When the temperature of a gas increases in a closed container, the pressure rises because particles move more rapidly and collide more frequently.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When gas is heated and particles race, understand it’s all about space!
Stories
Imagine a balloon. When you squeeze it, the air inside bumps against its walls harder, creating more pressure. When it's warm, it rushes, adding even more energy to the walls.
Memory Tools
Remember PVnRT: Picky Volumes need Ramps to Transition.
Acronyms
GAS
Gases Are Squishy - referring to their adaptability to volume.
Flash Cards
Glossary
- Ideal Gas Law
An equation of state for an ideal gas that relates pressure, volume, temperature, and moles of the gas.
- Kinetic Theory
A theory that explains the properties of gases in terms of the motion of their particles.
- Pressure
The force exerted by gas particles colliding with the walls of a container.
- Volume
The amount of space a gas occupies.
- Temperature
A measure of the average kinetic energy of particles in a substance.
- Mole
A unit that measures the amount of substance.
- Universal Gas Constant (R)
A constant used in the Ideal Gas Law, approximately equal to 8.314 J/(mol·K).
Reference links
Supplementary resources to enhance your learning experience.
