Energy Levels and Spectra
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Quantized Energy Levels
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are exploring how electrons exist in quantized energy levels. Can anyone tell me what we mean by 'quantized'?

It means they can only exist in specific, fixed levels, right?

Exactly, great job! Electrons can only inhabit certain energy levels, and transitions between these levels involve the absorption or emission of energy in the form of photons.

How do we calculate the energy of those photons?

Great question! We use the formula E=hf, where E is energy, h is Planck's constant, and f is the frequency of the photon. Can anyone help me remember what Planck's constant is?

It's 6.626 × 10^-34 J·s!

Correct! This constant is key in determining photon energy. Now, can someone summarize how energy levels affect electrons?

When electrons gain energy, they jump to a higher level, and when they lose energy, they fall to a lower level, right?

Yes! Well done. Energy levels dictate the behavior of electrons and influence the spectra we observe.
Emission Spectra
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, let's discuss emission spectra. What happens when an electron falls to a lower energy state?

It emits a photon!

Right again! This photon has energy corresponding to the difference between the two energy levels. What's significant about the wavelengths of these emitted photons?

They're unique to each element.

Exactly! That's why we can use emission spectra to identify elements. Can anyone give me an example of how this principle is applied?

In spectroscopy, right? We can determine what elements are present in stars.

Correct! And, in spectroscopy, these emissions can appear as lines of color on a spectrum, often referred to as line spectra. Let's summarize that emission spectra are useful for identifying elements based on these unique emissions.
Absorption Spectra
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand emission spectra, let's shift focus to absorption spectra. Who can explain what happens in this context?

When light passes through an atom, certain wavelengths are absorbed, creating dark lines in the spectrum.

Fantastic! The absorption spectrum essentially shows us which wavelengths were taken up by electrons advancing to higher energy levels. How does this differ visually from an emission spectrum?

The emission spectrum shows bright lines against a dark background, while the absorption spectrum shows dark lines against a continuous spectrum.

Correct! Understanding these differences is key in applications like astrophysics. Finally, can anyone summarize the importance of these spectra?

They help us identify elements and analyze the composition of substances.

Exactly! Excellent work, everyone.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
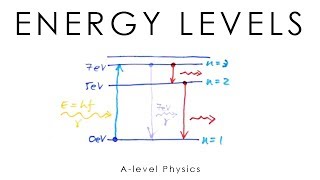
In this section, we explore the concept of quantized energy levels that electrons occupy within atoms. Transitions between these energy levels lead to the emission or absorption of photons, resulting in unique line spectra for each element. Both emission and absorption spectra are detailed, illustrating their significance in understanding atomic structure.
Detailed
Energy Levels and Spectra
In quantum mechanics, the energy levels of electrons in atoms are quantized, meaning they can only exist in predefined states rather than a continuous range. When absorbed or emitted, the energy difference between these levels corresponds to specific photon frequencies, described mathematically by the equation E=hf, where E is energy, h is Planck's constant, and f is frequency.
Emission Spectra
When electrons transition from higher energy levels to lower ones, they release energy in the form of photons, resulting in an emission spectrum. These spectra appear as characteristic lines of color unique to each element, which can be utilized to identify substances.
Absorption Spectra
Conversely, when atoms absorb photons that match the energy difference between energy levels, they create absorption spectra, which show dark lines within a continuous spectrum, indicative of which wavelengths are absorbed.
Understanding energy levels and the resulting spectra is crucial for applications in fields such as spectroscopy, astronomy, and chemical analysis.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Quantized Energy Levels
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Electrons occupy discrete energy levels. Transitions between these levels involve absorption or emission of photons with energy E=hf, where h is Planck's constant and f is the frequency.
Detailed Explanation
Electrons are not just found anywhere in an atom; they exist in specific, defined energy levels. This means that the energy of an electron is quantized, meaning it can only take on certain values. When electrons move between these levels, they either absorb or emit energy in the form of light, or photons. The amount of energy involved in these transitions can be calculated by the equation E=hf, where E is the energy of the photon, h is Planck's constant (a fundamental physical constant), and f is the frequency of the light. This principle is fundamental to understanding how atoms interact with light and is key to many areas of physics and chemistry.
Examples & Analogies
You can think of quantized energy levels like stairs in a building. Just as you can only stand on certain steps (not in between), electrons can only occupy specific energy levels. If you want to move from one floor to another (energy level), you have to use energy (just like jumping up or down the stairs). If light is absorbed, it’s like you taking a step up to a higher floor, and if light is emitted, it’s like you jumping down a step.
Emission Spectra
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When electrons drop to lower energy levels, they emit photons, producing characteristic line spectra unique to each element.
Detailed Explanation
When an electron in an atom moves from a higher energy level to a lower one, it releases energy in the form of a photon. This emitted photon has a specific wavelength and frequency that corresponds to the difference in energy between the two levels. As a result, each element emits a unique pattern of light, known as an emission spectrum, which appears as a series of lines at specific wavelengths. This is used in spectroscopy to identify elements in various substances.
Examples & Analogies
Imagine a musical note produced when a guitar string vibrates. Each note corresponds to a specific frequency. Similarly, when electrons drop energy levels, they produce ‘notes’ of light at specific wavelengths. Just as a musician can identify a song by the combination of notes played, scientists can identify different elements based on their unique emission spectra.
Absorption Spectra
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Atoms absorb photons corresponding to energy differences between levels, resulting in dark lines in the continuous spectrum.
Detailed Explanation
Absorption spectra occur when light passes through a gas or a vapor, and specific wavelengths are absorbed by the electrons in the atoms. When electrons absorb energy from photons, they jump to higher energy levels. The wavelengths of the absorbed light create dark lines on the continuous spectrum, corresponding to the energy differences between the levels. By analyzing these dark lines, scientists can determine the composition of distant stars and other celestial bodies.
Examples & Analogies
Think of absorption spectra like a colored filter that blocks certain colors of light. When you shine white light through a colored filter, some colors are absorbed, and others pass through. Similarly, when light passes through a cloud of gas, certain wavelengths are absorbed, creating dark lines. By examining which 'colors' are missing, scientists can determine what elements are present.
Key Concepts
-
Quantized Energy Levels: Electrons occupy discrete energy states within an atom.
-
Photon Emission: Electrons emit photons when transitioning to lower energy levels, creating emission spectra.
-
Photon Absorption: Electrons absorb photons when moving to higher energy levels, resulting in absorption spectra.
Examples & Applications
The bright lines seen in a helium emission spectrum help identify the presence of helium in stars.
The dark lines in the absorption spectrum of sunlight indicate the presence of various elements in the Sun's atmosphere.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In energy levels, they do reside, / Electrons jump, and photons glide.
Stories
Imagine an electron climbing a mountain (energy level) but returning down brings back a beautiful light (emission) while getting tired requires eating (absorption)!
Memory Tools
Remember E = hf: 'Every Photon is High-frequency light'.
Acronyms
PEA
Photon Emission Absorption (helps remember the key processes involved).
Flash Cards
Glossary
- Quantized Energy Levels
Discrete energy levels that electrons occupy in an atom, instead of a continuous range.
- Emission Spectrum
Spectrum of light emitted when electrons transition to lower energy levels, resulting in bright lines.
- Absorption Spectrum
Spectrum showing dark lines where light has been absorbed by electrons moving to higher energy levels.
- Photon
A particle of light that carries energy and is involved in electron transitions.
- Planck's Constant
A fundamental constant (6.626 × 10^-34 J·s) used in the calculation of energy of photons.
Reference links
Supplementary resources to enhance your learning experience.
