Historical Development of Atomic Models
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Dalton's Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start with Dalton's model of the atom. He proposed that atoms are indivisible particles. Does anyone know what that means?

It means atoms can't be divided into smaller parts, right?

Exactly! Dalton believed these indivisible atoms were the building blocks of all matter. This was revolutionary at the time because it suggested that everything around us is made of these tiny particles.

How did he know that? Did he have any experiments?

Great question! Dalton used careful measurements of chemical reactions to support his ideas. For example, he saw that elements combined in fixed proportions, which suggested the existence of atoms.

So, what came after Dalton?

Next, we moved on to Thomson’s model, which introduced the electron and changed our view of the atom! Remember his 'plum pudding' model?

Yeah, wasn't that where electrons were like plums in a positively charged pudding?

Exactly! The new concept of the electron was a huge advancement in understanding atomic structure.
Thomson's Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, after Dalton, J.J. Thomson discovered the electron. Can someone explain how this changed the atomic model?

He found that atoms have smaller particles inside them, right?

Correct! This led to the plum pudding model. But how did he discover the electron?

He used cathode rays, didn't he?

Exactly! He demonstrated that cathode rays were composed of negatively charged particles, which he named electrons.

What was wrong with the plum pudding model?

Good point! It didn’t account for the dense nucleus that would later be proposed by Rutherford.

So, then came Rutherford?

Correct! Through his gold foil experiment, he proved that atoms have a dense nucleus.
Rutherford's Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about Rutherford's model. After his gold foil experiment, what did he discover about the atom?

He found that most of an atom is empty space, and there's a concentrated center that is positively charged.

Exactly! He described a small, dense nucleus at the center with electrons surrounding it. Why was this significant?

It showed that atoms weren't just 'pudding' but had a structure!

Right! This new understanding set the stage for Bohr's model.

What did Bohr change?

Bohr introduced quantized energy levels for electrons. This was a leap because it helped explain atomic spectra.

So electrons are in set paths? How does that work?

Let's explore that in our next session!
Bohr's Model and Quantum Mechanical Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's discuss Bohr's model. Can someone summarize the key points?

Bohr said electrons occupy specific energy levels and can jump between them.

Exactly! These are quantized energy levels. Can anyone explain why this was important?

It helps explain why different elements emit different colors of light!

Very good! Those colors correspond to the photon energy emitted when electrons drop to lower energy levels. But there was still more to learn!

What about the Quantum Mechanical Model?

Excellent! Schrödinger and Heisenberg developed this, describing electrons in terms of probabilities, rather than fixed orbits. This was a major shift!

So instead of definite paths, we have regions of probability for where we might find an electron?

Exactly right! This model represents a fundamental change in how we understand atomic structure.

Can we summarize what we covered today?

Absolutely! Today we explored atomic models from Dalton, Thomson, and Rutherford to Bohr and finally the quantum mechanical model. Each one built on the last, giving us deeper insights into atomic structure.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section outlines the progression of atomic models, starting with Dalton's early description of the atom as indivisible, moving through Thomson's discovery of the electron, Rutherford's nuclear model, and Bohr's quantized orbits, culminating in the advanced quantum mechanical model developed by Schrödinger and Heisenberg which depicts electrons in terms of probabilities.
Detailed
Historical Development of Atomic Models
This section describes the significant milestones in the evolution of atomic models, which have profoundly shaped our understanding of atomic structure.
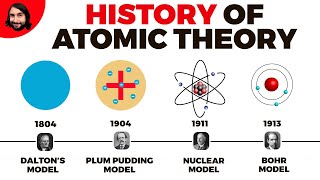
- Dalton's Model (Early 1800s): John Dalton proposed that atoms are indivisible particles that serve as the fundamental building blocks of matter. This early model laid the foundation for later theories about atomic composition.
- Thomson's Model (1897): J.J. Thomson discovered the electron in 1897, which led to the
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Dalton's Model (Early 1800s)
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Dalton's Model (Early 1800s): Proposed that atoms are indivisible particles, forming the fundamental building blocks of matter.
Detailed Explanation
John Dalton, an English chemist, introduced the idea of atoms in the early 1800s. He suggested that atoms are the smallest units of matter and cannot be divided further. This concept was crucial because it helped explain why substances combine in specific ratios to form compounds, laying the groundwork for modern chemistry.
Examples & Analogies
Think of atoms like LEGO blocks. Just as each LEGO block is a distinct piece that can fit together in certain ways to create different structures, atoms combine in specific ways to form everything around us.
Thomson's Model (1897)
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Thomson's Model (1897): Discovered the electron, leading to the "plum pudding" model where electrons are embedded within a positively charged sphere.
Detailed Explanation
In 1897, J.J. Thomson discovered the electron, which is a tiny, negatively charged particle. He proposed the 'plum pudding' model, which depicted the atom as a sphere of positive charge with electrons embedded in it, similar to plums in a pudding. This model suggested that atoms were more complex than Dalton proposed.
Examples & Analogies
Imagine a big bowl of pudding with bits of fruit mixed in. The pudding represents the positive charge of the atom, while the fruit pieces are the electrons scattered throughout it.
Rutherford's Model (1911)
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Rutherford's Model (1911): Through the gold foil experiment, concluded that atoms consist of a dense, positively charged nucleus surrounded by electrons.
Detailed Explanation
Ernest Rutherford conducted the famous gold foil experiment in 1911, where he directed alpha particles at a thin sheet of gold. He observed that while most particles passed through, some were deflected at large angles, leading him to conclude that atoms have a small, dense, positively charged nucleus at their center, surrounded by electrons. This was a significant shift from Thomson's model.
Examples & Analogies
Think of an atom like a small solar system. The nucleus is like the sun: dense and central, while the electrons are like planets orbiting around it.
Bohr's Model (1913)
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Bohr's Model (1913): Introduced quantized electron orbits, explaining atomic emission spectra.
Detailed Explanation
Niels Bohr presented his atomic model in 1913, integrating Rutherford’s nucleus with the new understanding of quantum mechanics. He proposed that electrons move in specific orbits or energy levels around the nucleus, and that they can jump between these levels, releasing or absorbing energy (light) in quantized amounts. This model explained why elements emit light at specific wavelengths.
Examples & Analogies
Picture a ladder where each step represents a specific energy level. Electrons can stand only on these steps, and when they jump from one to another, they release or absorb a specific amount of energy, like a sound emitted when a musician plays a note.
Quantum Mechanical Model
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Quantum Mechanical Model: Developed by Schrödinger and Heisenberg, this model describes electrons as wavefunctions, providing probability distributions for their positions.
Detailed Explanation
The Quantum Mechanical Model emerged from the work of physicists like Erwin Schrödinger and Werner Heisenberg. Instead of defining a clear path for electrons as in earlier models, this approach treats them as wavefunctions, which describe probabilities. This means we cannot pinpoint where an electron is exactly, but we can know where it's likely to be, represented as a 'cloud' around the nucleus.
Examples & Analogies
Imagine a crowd of people in a park. Instead of knowing where each person is standing at any moment, you can predict general areas where they might be based on where people usually gather. Similarly, the Quantum Mechanical Model gives us the probabilities of finding electrons in certain regions.
Key Concepts
-
Dalton's Atomic Model: Proposed atoms as indivisible particles.
-
Thomson's Plum Pudding Model: Suggested electrons within a positively charged sphere.
-
Rutherford's Nuclear Model: Introduced the concept of a dense nucleus.
-
Bohr's Model: Introduced quantized electron orbits.
-
Quantum Mechanical Model: Described electron positions using probabilities.
Examples & Applications
An example of Dalton's model is the assumption that chemical reactions involve rearranging atoms but not creating or destroying them.
Thomson’s model explains how cathode rays' deflection led to the discovery of electrons as negatively charged particles.
Rutherford’s gold foil experiment, which showed that most of the atom is empty space, leading to the concept of the atomic nucleus.
Bohr's approach to the hydrogen atom calculates energy levels and explains why the light spectrum shows specific lines.
The quantum mechanical model explains electron clouds, illustrating how electrons exist in a 'cloud of probability' around the nucleus.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Dalton said atoms can't split, Thomson's electrons, a tiny bit, Rutherford's core is a nucleus bright, Bohr’s circles lead to quantum light.
Stories
Imagine a baker (Dalton) who thought flour (atoms) couldn't be divided. Then a chef (Thomson) discovered that inside the cake (electron) were sweet layers, leading to a new recipe (Rutherford) that added a rich filling (nucleus). Finally, a scientist (Bohr) taught us how to bake in set temperatures (energy levels) to reach perfect confections (quantum behavior).
Memory Tools
DTRB: Dalton, Thomson, Rutherford, Bohr - the steps from indivisible to quantum!
Acronyms
EMERGE - Each Model Explains Real Growth of Electrons.
Flash Cards
Glossary
- Atom
The smallest unit of an element that retains the properties of that element.
- Electron
A negatively charged subatomic particle found in atoms.
- Nucleus
The dense central core of an atom, containing protons and neutrons.
- Quantized Energy Levels
Discrete levels of energy that electrons can occupy in an atom.
- Wavefunction
A mathematical description of the quantum state of a system.
Reference links
Supplementary resources to enhance your learning experience.
