Nature of Radioactive Emission
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Radioactive Emissions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore the nature of radioactive emissions. Can anyone tell me what they know about radioactivity?

I learned it has to do with unstable nuclei releasing energy.

Yeah, I think there are different types of emissions, right?

Exactly! There are three types: alpha, beta, and gamma. Let's start with alpha particles. Can anyone tell me what alpha particles are?

Are they the ones made of helium?

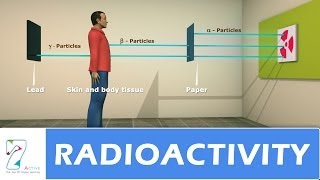
Correct! Alpha particles are actually helium nuclei, and they carry a positive charge of +2. Because of this charge, they have high ionizing power but low penetration capabilities. Does anyone remember what can stop alpha particles?

Paper stops them!

That's right! Now let’s summarize what we've learned about alpha particles. They are positively charged helium nuclei, have high ionizing power, and can be stopped by paper. Well done!
Understanding Beta Particles
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we have beta particles. Who can tell me what beta particles are?

I think they are electrons, right?

Yes! Beta particles are negatively charged electrons. They have moderate penetrating ability, which means they can go through materials like paper but can be stopped by aluminum. How does their ionizing power compare to alpha particles?

They have moderate ionizing power, so they are not as strong as alpha particles.

Exactly! While they are less ionizing than alpha particles, they still can have significant effects on matter. Remember, beta particles are electrons with moderate penetration and ionizing abilities.
Exploring Gamma Rays
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about gamma rays. Who knows what separates gamma rays from alpha and beta particles?

Are they not particles but electromagnetic waves?

Great observation! Gamma rays are indeed electromagnetic waves and carry no charge. They have very high penetrating power; in fact, they can pass through lead! But what can you tell me about their ionizing power?

Their ionizing power is low compared to alpha and beta particles.

Correct! Although gamma rays can penetrate many materials, they are less capable of ionizing them. To summarize: gamma rays are high-energy electromagnetic waves with high penetration abilities but low ionizing power.
Applications of Radioactive Emissions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the types of radioactive emissions, why do you think it's important to know about them?

Maybe because they have practical applications?

Exactly! Alpha particles, for example, have applications in smoke detectors, while beta particles are used in medical imaging. Gamma rays are particularly useful in cancer treatment and sterilization. Can anyone give me an example of a situation where each might be used?

Gamma rays in radiation therapy for cancer patients?

And beta particles for imaging like PET scans!

Excellent! Remember, understanding these radioactive emissions helps us harness their power safely and effectively in various fields.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the three types of radioactive emissions: alpha particles, which are helium nuclei with high ionizing power but low penetration; beta particles, which are electrons with moderate penetration and ionization; and gamma rays, which are high-energy electromagnetic waves with high penetration but low ionizing power.
Detailed
Nature of Radioactive Emission
In this section, we discuss the three primary types of radioactive emissions produced by unstable atomic nuclei: alpha (α) particles, beta (β) particles, and gamma (γ) rays. Each type of emission has distinct properties that delineate their nature and behavior in the environment.
- Alpha Particles (α):
- Composed of helium nuclei (4He).
- They are positively charged with a charge of +2.
- Alpha particles possess a low penetrating capability, being able to be stopped by a sheet of paper, but they have high ionizing power, making them capable of causing significant damage to other materials.
- Beta Particles (β):
- These are negatively charged electrons.
- They exhibit moderate penetration abilities and can be stopped by aluminum sheets.
- In terms of ionizing power, beta particles are considered to have a moderate impact.
- Gamma Rays (γ):
- Gamma rays are electromagnetic waves that carry no charge.
- They possess high penetrating power, able to pass through dense materials like lead, but they have a lower ionizing power compared to alpha and beta particles.
Understanding the nature of these radioactive emissions is crucial as it lays the foundation for comprehending the mechanisms behind radioactive decay and its applications in various fields including medicine, industry, and research.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Alpha (α) Particles
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Alpha (α) Particles:
● Positively charged (+2)
● Helium nuclei (24He^4_2He)
● Low penetration (stopped by paper)
● High ionizing power
Detailed Explanation
Alpha particles are a type of radioactive emission that carry a positive charge. Specifically, they are composed of helium nuclei, which means they consist of two protons and two neutrons. Due to their size and charge, alpha particles have low penetration abilities; they can be stopped by something as thin as a piece of paper. However, they have a high ionizing power, meaning they can easily ionize atoms they encounter, which can cause damage to living tissues if alpha sources are ingested or inhaled.
Examples & Analogies
Think of alpha particles as large, heavy balls. If you roll them towards a piece of paper, they'll easily be stopped. However, if these balls were to hit something fragile, they could cause a lot of damage, similar to how alpha radiation can impact biological tissues when ingested.
Beta (β) Particles
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Beta (β) Particles:
● Negatively charged electrons (−10e^0_{-1}e)
● Moderate penetration (stopped by aluminum)
● Moderate ionizing power
Detailed Explanation
Beta particles are essentially high-speed electrons emitted from radioactive decay. They carry a negative charge, which allows them to interact with matter somewhat differently than alpha particles. Beta particles have moderate penetration capability; they can penetrate materials better than alpha particles but can be stopped by a layer of aluminum. Their ionizing power is also moderate, which means they can still cause damage to cells but typically not as severely as alpha particles.
Examples & Analogies
Imagine beta particles as small, fast-moving tennis balls. They can travel further than the heavier alpha particles, and if they hit something like an aluminum sheet, they will bounce off and not go through. While they are not as damaging as alpha particles, they can still cause a bit of a ruckus if they hit living cells, similar to how a fast tennis ball can cause a bruise.
Gamma (γ) Rays
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Gamma (γ) Rays:
● Electromagnetic waves (no charge)
● High penetration (can pass through lead)
● Low ionizing power
Detailed Explanation
Gamma rays are a form of electromagnetic radiation, similar to visible light but with much higher energy. Unlike alpha and beta emissions, gamma rays are neutral and have no charge, which allows them to penetrate materials very effectively. They can pass through thick lead shielding but have lower ionizing power compared to alpha and beta particles, meaning they interact less frequently with matter. This makes them less likely to ionize atoms directly, but they can still cause damage through secondary reactions.
Examples & Analogies
Think of gamma rays as invisible, high-energy lasers. They can easily go through walls and even lead, which is why they need heavy shielding to protect against them. While they don't cause immediate damage like heavier particles, they can still affect things around them, just as an intense laser can burn things even without making contact.
Key Concepts
-
Alpha Particles: Helium nuclei that are positively charged, low penetration but high ionizing power.
-
Beta Particles: Negatively charged electrons, moderate penetration and ionizing power.
-
Gamma Rays: Electromagnetic waves with high penetration abilities but low ionizing power.
Examples & Applications
Alpha particles are used in smoke detectors due to their high ionizing ability.
Beta particles are utilized in medical imaging technologies like PET scans.
Gamma rays are applied in cancer treatment through targeted radiation therapy.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Alpha's a particle with a charge that's high, / Stopped by paper, it’s not shy.
Stories
Once upon a time, there were three friends: Alpha, Beta, and Gamma. Alpha was strong but couldn’t go through paper. Beta was nimble, able to sneak through aluminum. Gamma was the strongest of them all, passing through lead walls without a call!
Memory Tools
A useful mnemonic for remembering the types of radiation is ‘All Bright Girls’: A for Alpha, B for Beta, G for Gamma.
Acronyms
To remember the properties of emissions, use the acronym 'P-I-P', which stands for Particle type (α, β, γ), Ionizing power (high, moderate, low), and Penetration ability (paper, aluminum, lead).
Flash Cards
Glossary
- Alpha Particle
A positively charged particle made up of two protons and two neutrons, equivalent to a helium nucleus.
- Beta Particle
A negatively charged electron emitted from a nucleus during radioactive decay.
- Gamma Ray
A high-energy electromagnetic wave emitted by a radioactive nucleus.
Reference links
Supplementary resources to enhance your learning experience.
