Formation of Covalent Compounds (Examples)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Covalent Bond Basics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing how covalent compounds are formed. Does anyone remember what a covalent bond is?

Is it when atoms share electrons?

Exactly! Atoms share electrons to achieve a full outer shell. Who can tell me why this sharing happens?

To have a stable electronic configuration, like noble gases?

Correct! Remember the octet rule. Shared electrons help both atoms become more stable. Now, let's dive into our first example, water.
Water Formation (H₂O)
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Water is a classic example of a covalent compound. What atoms make up water?

Two hydrogen atoms and one oxygen atom!

Right! Now, oxygen has six valence electrons. How many does it need to share to be stable?

It needs to share two more electrons!

Correct! So, it shares electrons with two hydrogen atoms. Each hydrogen shares its one electron, allowing oxygen to complete its octet. Can anyone summarize what we've just learned?

Oxygen shares two electrons with hydrogen, making water a covalent compound.
Methane Formation (CH₄)
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's look at methane, CH₄. What can someone tell me about its formation?

It has one carbon and four hydrogen atoms!

Exactly! Carbon has four valence electrons. How does it bond with hydrogen?

It shares all four of its electrons with four hydrogen atoms.

Yes! This sharing forms four covalent bonds, ensuring that all atoms achieve stability. Can anyone explain why methane is stable?

Because carbon and hydrogen atoms complete their outer shells by sharing electrons!
Summary of Covalent Bonding
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To wrap up, what are the key points we learned today about covalent compounds?

Covalent bonds involve sharing electrons between non-metals.

Water and methane are both examples where atoms share electrons to become stable.

Great recaps! Remember, covalent bonding is essential not only for understanding simple molecules but also more complex structures as we advance in chemistry.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section elaborates on the formation of covalent compounds by exploring how atoms share electrons to achieve stable electronic configurations. Using water (H₂O) and methane (CH₄) as practical examples, it illustrates how these compounds are formed through the sharing of valence electrons between different non-metals.
Detailed
Formation of Covalent Compounds (Examples)
Covalent compounds are formed when two or more non-metal atoms share electrons. This type of bonding enables each atom to achieve a stable electronic configuration, often described by the octet rule.
Key Examples:
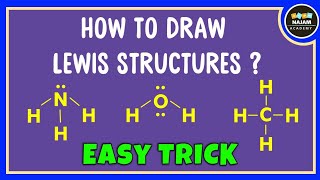
- Water (H₂O): In the formation of water, the oxygen atom shares its six valence electrons with two hydrogen atoms, creating two covalent bonds. This allows oxygen to complete its valence shell with eight electrons, while each hydrogen achieves a stable configuration with two electrons.
- Methane (CH₄): For methane, one carbon atom shares its four valence electrons with four hydrogen atoms, forming four covalent bonds. This arrangement allows both carbon and hydrogen to reach stable electronic states.
Understanding covalent bonding is crucial in chemistry as it lays the foundation for grasping more complex molecular structures and reactions.
Youtube Videos

![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Water (H₂O)
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Water (H₂O):
○ Oxygen shares electrons with 2 Hydrogen atoms.
Detailed Explanation
In the formation of a water molecule (H₂O), the oxygen atom shares its electrons with two hydrogen atoms. Oxygen has six valence electrons and needs two more to complete its outer shell (octet). Each hydrogen atom has one valence electron and needs one to have a complete outer shell. By sharing, all atoms achieve a stable electron configuration, leading to the formation of a covalent bond.
Examples & Analogies
Think of it as a partnership where oxygen and hydrogen are two friends that need support to feel complete. By sharing their 'resources' (electrons), they help each other out and create a strong bond, just like friends help one another in life.
Methane (CH₄)
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Methane (CH₄):
○ Carbon shares its 4 valence electrons with 4 Hydrogen atoms.
Detailed Explanation
In methane, the carbon atom has four valence electrons and can form four bonds. Each of the four hydrogen atoms has one valence electron. By sharing its electrons with the four hydrogen atoms, carbon achieves a complete outer shell, and each hydrogen also completes its shell. This results in four covalent bonds in the methane molecule, providing it with stability.
Examples & Analogies
Imagine carbon as a host at a dinner party, and each hydrogen atom is a guest. The carbon host shares its resources (electrons) equally with the guests to ensure that everyone is happy and satisfied. This sharing creates a harmonious gathering, similar to how the covalent bonds make up a stable methane molecule.
Key Concepts
-
Covalent Bond: A bond formed by sharing electrons between non-metals.
-
Shared Electrons: Electrons that two atoms share to form covalent bonds.
-
Stable Electronic Configuration: The state achieved by atoms when their outermost shell is full.
Examples & Applications
Water (H₂O) where oxygen shares electrons with two hydrogen atoms.
Methane (CH₄) where carbon shares four valence electrons with four hydrogen atoms.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Covalent bonding, oh so sweet, / Atoms share to feel complete!
Stories
Once upon a time, in a world of tiny atoms, oxygen was looking for friends. It found two lonely hydrogen atoms. Together, they shared their electrons to form water. Along the way, they discovered how sharing made them much happier and stable.
Memory Tools
HOMES for water H₂O: Hydrogen, Oxygen, Molecule, Electron Sharing.
Acronyms
SHARE
Stable Hydrogen Atoms Result from Electrons.
Flash Cards
Glossary
- Covalent Bond
A chemical bond formed by the sharing of electrons between two non-metal atoms.
- Valence Electrons
Electrons in the outermost shell of an atom, crucial for determining how an atom bonds with others.
- Octet Rule
The principle that atoms tend to bond in such a way that they each have eight electrons in their valence shell.
- Molecular Compound
A chemical compound whose simplest units are molecules.
Reference links
Supplementary resources to enhance your learning experience.
