Properties of Ionic and Covalent Compounds
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Formation and Physical State of Ionic vs. Covalent Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start by discussing the formation of ionic and covalent compounds. Can anyone tell me how ionic compounds are formed?

I think they form when one atom gives electrons to another atom.

Correct! Ionic compounds are created through electron transfer, usually between a metal and a non-metal. What about covalent compounds? How are they formed?

They form by sharing electrons between two non-metals.

Exactly! Now, can someone tell me the physical states that ionic and covalent compounds typically exist in?

Ionic compounds are usually solid, right?

And covalent compounds can be liquids or gases.

Great observations! To summarize, ionic compounds are solid due to strong ionic bonds, whereas covalent compounds can exist in liquid or gas form due to weaker forces between molecules.
Melting and Boiling Points of Ionic and Covalent Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss melting and boiling points. Why do you think ionic compounds have high melting and boiling points?

Because of the strong forces between the ions?

Exactly! The electrostatic forces hold the ions tightly together. Now, what about covalent compounds? Why do they tend to have lower melting and boiling points?

Because the forces between molecules are weaker compared to ionic bonds?

Right again! To recap, ionic compounds have high melting and boiling points due to strong ionic bonds, while covalent compounds are generally lower because of weaker intermolecular forces.
Solubility and Electrical Conductivity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s talk about solubility. How do ionic compounds behave when placed in water?

They dissolve and dissociate into ions.

Exactly! And what about covalent compounds? Are they generally soluble in water?

Most of them are not very soluble in water.

Correct! Now let’s link this to electrical conductivity. How do ionic compounds conduct electricity?

They conduct when dissolved in water or melted because the ions can move freely.

That's right! In contrast, why don’t covalent compounds conduct electricity?

Because they don’t have free-moving ions or charged particles.

Excellent summary! This wraps up our discussion on the properties of ionic and covalent compounds.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Ionic and covalent compounds exhibit distinct properties based on their formation mechanisms. Ionic compounds are typically solid, have high melting and boiling points, are soluble in water, and can conduct electricity in molten or solution form. In contrast, covalent compounds are often liquid or gas, tend to have lower melting and boiling points, are generally insoluble in water, and do not conduct electricity.
Detailed
Properties of Ionic and Covalent Compounds
This section focuses on distinguishing characteristics of ionic and covalent compounds, essential for understanding their behavior in different contexts.
Properties of Ionic Compounds
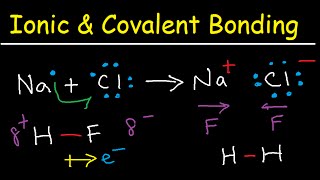
- Formation: Ionic compounds are formed by the transfer of electrons, usually between metals and non-metals. One atom loses electrons to form a cation while another gains electrons to form an anion.
- Physical State: They are usually found as solids, forming crystal lattices that give them structural integrity.
- Melting and Boiling Points: Ionic compounds have high melting and boiling points due to the strong electrostatic forces between the oppositely charged ions.
- Solubility in Water: These compounds are typically soluble in water, dissociating into their constituent ions.
- Electrical Conductivity: Ionic compounds can conduct electricity when melted or dissolved in water, as the ions are free to move.
Properties of Covalent Compounds
- Formation: Covalent compounds are formed by the sharing of electrons between non-metals.
- Physical State: They often exist as liquids or gases at room temperature.
- Melting and Boiling Points: Covalent compounds tend to have lower melting and boiling points compared to ionic compounds.
- Solubility in Water: Many covalent compounds are generally insoluble in water.
- Electrical Conductivity: These compounds do not conduct electricity, as they lack free-moving charged particles.
Understanding these properties is fundamental in predicting the behavior of substances in chemical reactions and their applications in various fields.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Formation of Ionic Compounds
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Formation
- Ionic Compounds: Formed by transfer of electrons.
Detailed Explanation
Ionic compounds are created when one atom gives up electrons, and another atom takes those electrons. This transfer creates charged particles known as ions. The atom that loses electrons becomes positively charged (cation), and the one that gains electrons becomes negatively charged (anion). The attraction between these oppositely charged ions forms the ionic bond.
Examples & Analogies
Think of ionic bonding like a game of catch. One person throws a ball (an electron) to another. Once the ball is caught, the receiver must keep it (the electron), and the thrower has to understand that now they are missing the ball. The excitement of playing together makes both players stay engaged, similar to how ions stay together due to their opposite charges.
Formation of Covalent Compounds
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Formation
- Covalent Compounds: Formed by sharing of electrons.
Detailed Explanation
Covalent compounds are created when two or more atoms share electrons. This sharing allows each atom to achieve stability by filling their outer electron shell. Unlike ionic compounds formed by the transfer of electrons, covalent bonds focus on mutual cooperation between atoms to reach a stable state.
Examples & Analogies
Consider covalent bonding like a partnership in a project. Two friends (atoms) come together to complete a project (achieve stability). To do this, they each bring materials (electrons) to use and combine their resources, which helps them finish the project together successfully and fulfill their needs.
Physical State of Compounds
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Physical State
- Ionic Compounds: Usually solids.
- Covalent Compounds: Usually liquids or gases.
Detailed Explanation
Ionic compounds commonly exist as solid structures due to the strong attraction of the ions within the lattice formation. This tightly bound structure is why ionic compounds tend to be hard and brittle. In contrast, covalent compounds exhibit weaker intermolecular forces, allowing many to exist as liquids or gases at room temperature.
Examples & Analogies
Think of ionic compounds as a solid fortress with walls made by tightly packed soldiers (ions), while covalent compounds are more like a casual group of friends hanging out, where they can easily shift positions depending on their environment (solid, liquid, or gas).
Melting and Boiling Points
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Melting/Boiling Points
- Ionic Compounds: High.
- Covalent Compounds: Low.
Detailed Explanation
Ionic compounds feature stronger ionic bonds requiring significant energy to break, resulting in high melting and boiling points. In contrast, covalent compounds have weaker intermolecular forces, making them easier to lose their structural integrity at lower temperatures.
Examples & Analogies
Imagine trying to break a thick rope (ionic) compared to a thin string (covalent). The rope is tough and hard to break, requiring more effort (energy) compared to the string, which can snap much easier at a lower tension (temperature).
Solubility in Water
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Solubility in Water
- Ionic Compounds: Soluble.
- Covalent Compounds: Generally insoluble.
Detailed Explanation
Ionic compounds tend to dissolve in water because the polar water molecules can effectively separate the ions from one another. On the other hand, many covalent compounds do not dissolve in water since they lack ionic character and cannot interact powerfully with water molecules.
Examples & Analogies
Think of ionic compounds as sugar dissolving in coffee — it breaks apart and mixes well. In contrast, oil is like covalent compounds that don’t mix with water; they remain separate even when stirred, illustrating their general insolubility.
Electrical Conductivity
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Electrical Conductivity
- Ionic Compounds: Conduct in molten/solution.
- Covalent Compounds: Do not conduct.
Detailed Explanation
Ionic compounds can conduct electricity when they are melted or dissolved in water due to the mobility of their ions. Covalent compounds typically do not have charged particles that can move freely, so they do not conduct electricity.
Examples & Analogies
Think of ionic compounds as a highway where cars (ions) can travel freely when the road is clear (when dissolved in water). In contrast, covalent compounds are like traffic stuck on a narrow street where no cars can pass through, making it impossible for movement (electricity) to occur.
Key Concepts
-
Ionic Compounds: Formed by electron transfer and typically solid with high melting points.
-
Covalent Compounds: Formed by electron sharing, usually liquid or gas at room temperature, with lower melting points.
Examples & Applications
Sodium Chloride (NaCl) is an example of an ionic compound, while water (H₂O) is an example of a covalent compound.
Ionic compounds like MgO have high melting points, while covalent compounds like CO₂ have lower melting points.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ionic's solid and strong, melts high, stands long; Covalent flows like a song, low melts, in water, not belong.
Stories
Once in a school of chemistry, there were two sets of friends: the Ionic kids, solid and sturdy, who stood tall and never melted down; and the Covalent kids, who danced fluidly, flowing together but could never form a hard line.
Memory Tools
For Ionic, think 'Ionic Is Strongly Solid', and for Covalent, recall 'Covalent Can Change' (liquid/gas).
Acronyms
Remember 'MSE SED' for properties
'M' for Melting points high for ionic
'S' for Solubility in water
'E' for Electrical conductivity; 'S' for state liquid/gas for covalent.
Flash Cards
Glossary
- Ionic Compound
A chemical compound formed by the transfer of electrons from one atom to another, resulting in electrostatic attraction between oppositely charged ions.
- Covalent Compound
A chemical compound formed by the sharing of electrons between atoms, typically between non-metals.
- Melting Point
The temperature at which a solid becomes a liquid.
- Boiling Point
The temperature at which a liquid becomes a gas.
- Electrostatic Forces
Attractive forces between charged particles, such as ions.
- Solubility
The ability of a substance to dissolve in a solvent.
Reference links
Supplementary resources to enhance your learning experience.
