Hydrogen
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Hydrogen
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re going to explore hydrogen, the first element on the periodic table. Can anyone tell me its atomic number?

Is it 1?

Exactly! Hydrogen has an atomic number of 1 and an atomic mass of about 1.008 u. It exists in nature as a diatomic molecule, represented as H₂.

Why is it called hydrogen?

Great question, Student_2! The name 'hydrogen' comes from the Greek words 'hydro' meaning water and 'genes' meaning creator. Hydrogen is essential for forming water.

Is hydrogen common in space?

Yes, it is the most abundant element in the universe! It’s found in stars, including our Sun.

What about Earth? Is it common here too?

Hydrogen is found in compounds like water, but it’s rare in its free form due to its lightness and tendency to escape into the atmosphere. Let’s remember that H2O represents both hydrogen and oxygen!
Properties of Hydrogen
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive into hydrogen’s properties. Who can describe its physical characteristics?

It’s a gas that’s colorless and odorless!

That's right! Hydrogen also burns with a pale blue flame. Can anyone tell me its solubility in water?

Is it slightly soluble?

Exactly! Now, moving on to chemical properties, hydrogen combusts in oxygen to form water. Can someone give me the reaction equation?

2H₂ + O₂ → 2H₂O!

Perfect! This reaction is exothermic, which means it releases energy. Hydrogen also reduces metal oxides to their respective metals. For example, what happens with CuO?

It turns into copper and water!

Correct! Excellent understanding of hydrogen’s properties.
Isotopes of Hydrogen
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s talk about isotopes of hydrogen. Can anyone name the three isotopes?

Protium, Deuterium, and Tritium!

Excellent! Protium is the most common isotope, making up around 99.98%. What about Deuterium and Tritium?

Deuterium has one neutron and Tritium has two! Tritium is radioactive.

Exactly! Deuterium is used in nuclear reactors while Tritium has applications in research because of its radioactivity.
Uses of Hydrogen
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's explore the uses of hydrogen. Can someone name where hydrogen is commonly used?

In welding with an oxy-hydrogen flame!

That’s right! Hydrogen is also essential in the Haber process for producing ammonia. Can anyone mention another application?

It’s used in fuel cells for generating electricity!

Perfect! Remember, hydrogen is viewed as a clean fuel for the future since it only produces water when burned. Isn’t that fascinating?
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Hydrogen, the lightest element and first on the periodic table, is essential for life and has numerous industrial applications. This section explores its occurrence in nature, its unique position in the periodic table, various methods for its preparation, physical and chemical properties, its isotopes, and its critical importance as a clean energy source and in various industries.
Detailed
Detailed Overview of Hydrogen
Hydrogen (H), with an atomic number of 1, is the first element on the periodic table and the most abundant element in the universe. It primarily exists as a diatomic molecule (H) in nature and is a key component of essential compounds such as water (HO) and organic matter. Although hydrogen is rare in its free form on Earth due to its lightness, it is found in various natural sources, including the Sun, stars, and volcanic gases.
Hydrogen’s unique position on the periodic table exhibits properties that are characteristic of both alkali metals and halogens. It possesses one valence electron and can form +1 ions similar to alkali metals, while also forming covalent bonds like halogens.
The preparation of hydrogen can occur through several methods, notably through the reaction of zinc and dilute hydrochloric acid, with careful collection procedures due to its flammability. The characteristics of hydrogen as a colorless, odorless, and tasteless gas that burns with a pale blue flame are contrasted next to its crucial chemical properties, including its combustion with oxygen to produce water and its role in reducing metal oxides.
Hydrogen’s applications range from being a crucial component in welding to its important role in the Haber process for ammonia production and as rocket fuel. It stands out due to its isotopes: Protium, Deuterium, and Tritium, each with distinct properties and uses. As a clean fuel source, hydrogen holds immense potential for the future, particularly in the energy sector.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Hydrogen
Chapter 1 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Hydrogen (H) is the first and lightest element in the periodic table.
● Atomic number: 1
● Atomic mass: 1.008 u
● Exists as a diatomic molecule (H₂) in nature.
Detailed Explanation
Hydrogen, represented by the symbol H, is the simplest and lightest element in the periodic table. It has an atomic number of 1, which means it has one proton in its nucleus. Its atomic mass is approximately 1.008 atomic mass units (u). In nature, hydrogen is predominantly found as a diatomic molecule (H₂), meaning that two hydrogen atoms bond together to form a molecule.
Examples & Analogies
Think of hydrogen like the smallest building block of matter. If you consider a Lego set, hydrogen is like a single Lego piece. Just as many legos can be combined to build larger structures, many hydrogen atoms combine to form various compounds, including the water we drink.
Occurrence of Hydrogen
Chapter 2 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Most abundant element in the universe.
● Found in:
○ Water (H₂O)
○ Organic compounds
○ Sun and stars
○ Volcanic gases
● Rare in free form on Earth due to its lightness.
Detailed Explanation
Hydrogen is the most plentiful element in the universe, playing a crucial role in the composition of many celestial bodies. On Earth, hydrogen is primarily found in compounds rather than in its free form. It is a key component of water (H₂O) and organic compounds that make up living organisms. Hydrogen is also present in the sun and stars, where it undergoes nuclear fusion. However, due to hydrogen's lightness, it escapes Earth's atmosphere, making it rare to find in its uncombined state on Earth.
Examples & Analogies
Imagine a balloon filled with helium. Just as the helium floats away into the sky because it is lighter than air, hydrogen does the same. If you think of Earth as a balloon filled with gases, most of the light gases like hydrogen tend to float away and escape into space, leaving it mostly bonded in compounds.
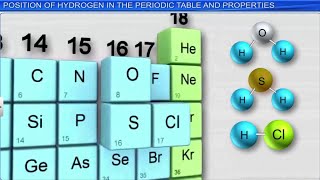
Position in the Periodic Table
Chapter 3 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Unique placement: Shows properties of both Group 1 (alkali metals) and Group 17 (halogens).
● Like alkali metals: Has 1 valence electron and forms +1 ions.
● Like halogens: Forms covalent bonds and diatomic molecules.
Detailed Explanation
Hydrogen occupies a unique position in the periodic table, where it exhibits characteristics of both alkali metals (Group 1) and halogens (Group 17). Like alkali metals, hydrogen has one valence electron, allowing it to easily lose that electron and form a +1 ion. However, it also shares properties with halogens, as it can form diatomic molecules (H₂) and covalent bonds with non-metals.
Examples & Analogies
Think of hydrogen as a versatile actor in a play. Just as an actor can perform different roles to fit different characters, hydrogen can adapt to behave like either an alkali metal or a halogen, depending on the chemical context it finds itself in.
Preparation of Hydrogen
Chapter 4 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Laboratory Method:
○ By reaction of zinc with dilute hydrochloric acid:
■ Zn + 2HCl → ZnCl₂ + H₂↑
○ Collected by downward displacement of water as it is insoluble in water and lighter than air.
● Precautions:
○ Use dilute acid only.
○ Start collecting gas after air is displaced.
Detailed Explanation
In laboratory settings, hydrogen gas can be prepared by reacting zinc (Zn) with dilute hydrochloric acid (HCl). This reaction produces zinc chloride (ZnCl₂) and hydrogen gas (H₂). Since hydrogen is lighter than air and insoluble in water, it can be collected using the downward displacement of water method. It's important to take safety precautions, such as using only dilute acid to prevent violent reactions and ensuring that all air is displaced before collecting the gas.
Examples & Analogies
Think of making lemonade; if you add too much sugar at once, it can make the drink overly sweet. Similarly, using concentrated acid can lead to an uncontrolled reaction. Just like making lemonade requires careful mixing and patience, preparing hydrogen in the lab involves understanding the right proportions and waiting for the air to clear before capturing the gas.
Properties of Hydrogen
Chapter 5 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Physical Properties:
○ Colorless, odorless, tasteless gas.
○ Lightest known element.
○ Slightly soluble in water.
○ Burns with a pale blue flame.
● Chemical Properties:
○ Combustion: Burns in oxygen to form water.
2H₂ + O₂ → 2H₂O (exothermic)
○ Reduces metal oxides to metals:
■ CuO + H₂ → Cu + H₂O
○ Forms covalent compounds with non-metals:
■ H₂ + Cl₂ → 2HCl
Detailed Explanation
Hydrogen has several interesting properties. Physically, it is a colorless, odorless, and tasteless gas, making it hard to detect without specific instruments. It is the lightest known element and can slightly dissolve in water. When hydrogen burns in oxygen, it produces water, which is an exothermic reaction, meaning it releases heat. Chemically, hydrogen can reduce metal oxides to their respective metals and can also form covalent bonds with non-metals, as illustrated by the reactions with copper oxide and chlorine.
Examples & Analogies
Imagine hydrogen like a quiet whisper in a crowded room. Its invisibility (colorless, odorless, and tasteless) makes it sneak past unnoticed until it bursts into flame (burning with a pale blue flame) when it meets oxygen. This reaction creates water, akin to a secret transforming into something beautiful and essential, like rain nourishing the earth.
Uses of Hydrogen
Chapter 6 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Used in welding (oxy-hydrogen flame).
● Production of ammonia (Haber process).
● Hydrogenation of oils to make ghee.
● Rocket fuel.
● In fuel cells for electricity generation.
Detailed Explanation
Hydrogen has a wide range of applications in various industries. It is commonly used in welding, where an oxy-hydrogen flame provides the high temperature needed to join metals. Additionally, hydrogen plays a crucial role in the Haber process, which produces ammonia for fertilizers. It is also used in the hydrogenation of oils to solidify them into ghee and serves as rocket fuel due to its high energy output. Moreover, hydrogen fuel cells are becoming increasingly popular as a clean energy source for electricity generation.
Examples & Analogies
Think of hydrogen as a swiss army knife in industrial applications. Just like a swiss army knife has various tools for different tasks, hydrogen has multiple uses, from helping build structures with welding to feeding the world through ammonia production, showcasing its versatility in many fields.
Isotopes of Hydrogen
Chapter 7 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Hydrogen has three isotopes:
Isotope Symbol Proton Neutron Natural Abundance
Protium ¹H 1 0 ~99.98%
Deuterium ²H or D 1 1 ~0.02%
Tritium ³H or T 1 2 Very rare
● Deuterium is used in nuclear reactors.
● Tritium is radioactive and used in research.
Detailed Explanation
Hydrogen exists in three isotopes—Protium (¹H), Deuterium (²H), and Tritium (³H). Protium, the most common isotope, makes up about 99.98% of all hydrogen and has one proton with no neutrons. Deuterium contains one proton and one neutron, while Tritium is rarer and contains one proton and two neutrons, making it radioactive. Deuterium is often used in nuclear reactors, while Tritium has applications in research due to its radioactive properties.
Examples & Analogies
Think of isotopes like different flavors of ice cream. While all the flavors are essentially ice cream (like how all isotopes are hydrogen), each flavor has its own unique taste and characteristics. Protium is like vanilla, the most popular flavor, while Deuterium is a bit more special like cookies and cream, and Tritium is rare like a limited-edition flavor available only at exclusive shops.
Importance of Hydrogen
Chapter 8 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Essential for life—found in water and organic molecules.
● Key element in energy and chemical industries.
● Seen as a clean fuel of the future (produces only water on combustion).
Detailed Explanation
Hydrogen is vital for life on Earth as it constitutes water and organic molecules that make up living organisms. Moreover, it is an essential element in various chemical and energy industries. In the context of environmental concerns, hydrogen is increasingly recognized as a clean fuel of the future because burning it produces only water as a byproduct, which could significantly reduce pollution.
Examples & Analogies
Consider hydrogen as the backbone of life’s recipe. Just as a good recipe requires key ingredients, hydrogen is crucial for the recipe of life, providing the essential elements found in water and organic compounds. As we move toward a greener future, think of hydrogen as the ingredient we need to create a cleaner, more sustainable world.
Key Concepts
-
Hydrogen: The first and lightest element essential in forming water and organic compounds.
-
Occurrence: Most abundant in the universe but rare in its elemental form on Earth.
-
Properties: Hydrogen is colorless, odorless, and burns with a pale blue flame.
-
Isotopes: Hydrogen has three isotopes: Protium, Deuterium, and Tritium, with varying abundances and uses.
-
Applications: Used in welding, ammonia production, fuel cells, and recognized as a clean fuel source.
Examples & Applications
Hydrogen's reaction with oxygen produces water (2H₂ + O₂ → 2H₂O).
Hydrogenation involves adding hydrogen to unsaturated fats, converting oils to ghee.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hydrogen's light, it brings the night, in water it's found, safe and sound.
Stories
Once upon a time, in the stars above, lived hydrogen, the lightest, known for its love. It danced in water, laughed in the sun, in fuels and plants, it made life fun!
Memory Tools
H2O – Hydrogen plus Oxygen equals water; think of H2 = Happiness to remember hydrogen's importance!
Acronyms
HAVE FUN – Hydrogen's Abundance; Vital for energy, Fuel, and Universal Nature.
Flash Cards
Glossary
- Diatomic Molecule
A molecule composed of two atoms, such as H₂ for hydrogen.
- Isotope
Different forms of the same element that contain equal numbers of protons but different numbers of neutrons.
- Hydrogenation
A chemical reaction that adds hydrogen to a compound, often used in the food industry.
- Exothermic Reaction
A reaction that releases energy in the form of heat or light.
- Haber Process
An industrial method for synthesizing ammonia from nitrogen and hydrogen.
- Nuclear Reactor
A device that initiates and controls a sustained nuclear chain reaction.
Reference links
Supplementary resources to enhance your learning experience.
