Introduction to Hydrogen
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Properties and Basics of Hydrogen
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're discussing hydrogen, the first element in the periodic table. Can anyone tell me what its atomic number is?

Is it 1?

Exactly! Hydrogen has an atomic number of 1. It’s also the lightest element. What form does it exist in nature?

I think it exists as H₂, right?

Correct! Hydrogen naturally occurs as a diatomic molecule, H₂. This means two hydrogen atoms are bonded together. This is important for how it interacts chemically. Can anyone remember other key properties of hydrogen?

It’s colorless and odorless!

Great point! Hydrogen is indeed colorless, odorless, and tasteless. Remember that it burns with a pale blue flame, a detail that can help you visualize its properties.

Why is it the most abundant element?

Good question! Hydrogen is the most abundant element in the universe, primarily found in stars, including our sun, and is present in water here on Earth. Let’s summarize: Hydrogen's atomic number is 1, it’s a diatomic molecule (H₂), it's colorless and burns with a pale blue flame.
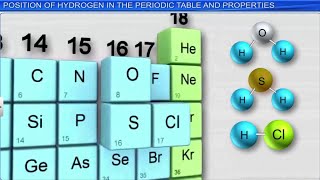
Hydrogen's Position in the Periodic Table
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s delve into hydrogen's position in the periodic table. Does anyone know what groups it resembles?

It looks like Group 1 and Group 17?

Exactly! Hydrogen sits at the top and displays properties similar to both Group 1, the alkali metals, and Group 17, the halogens. What’s interesting is that it forms +1 ions like alkali metals but also shares the ability to form covalent bonds like halogens.

So it’s kind of in a category of its own?

Yes! Its unique characteristics make it versatile in reactions. Can anyone think of an example of hydrogen in a chemical reaction?

It reacts with oxygen to form water!

Correct! H₂ + O₂ → H₂O demonstrates hydrogen's reactivity. Summarizing today: hydrogen resembles both alkali metals and halogens due to its valency and bond-forming capabilities.
Occurrence and Abundance of Hydrogen
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss where hydrogen can be found. Who knows the major sources of hydrogen?

I think it’s in water, in organic compounds, and in stars?

Absolutely! Hydrogen is found in water (H₂O) and is also a part of organic compounds, making it essential for life. Its abundance in the universe is primarily in stars and is generated in volcanic gases on Earth.

Why is there so little hydrogen on Earth?

Good observation! Due to its low molecular weight, hydrogen is rare in its free form in Earth's atmosphere. What’s the implication of this?

So it's mostly found combined with other elements?

Yes, that's right! To summarize: Hydrogen is abundant in the universe, primarily forming H₂O and organic compounds but rarely found free on Earth.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Hydrogen, with an atomic number of 1 and atomic mass of 1.008 u, is fundamental to both the universe's composition and various aspects of chemical science. Its occurrence as a diatomic molecule (H₂) and its unique placement in the periodic table, as well as its combination of properties from both alkali metals and halogens, highlight its versatile role in nature and industry.
Detailed
Introduction to Hydrogen
Hydrogen is the simplest element, represented by the symbol H, and has an atomic number of 1 and an atomic mass of approximately 1.008 u. It naturally occurs as a diatomic molecule (H₂), meaning two hydrogen atoms bond together. Hydrogen is not only the lightest element but also the most abundant one in the universe, finding essential roles in water (H₂O), organic compounds, and celestial bodies like the sun and stars.
Properties and Positioned in the Periodic Table
Hydrogen's unique position in the periodic table reflects its dual characteristics. While it shares similarities with alkali metals due to its single valence electron, which allows it to form +1 ions, it also exhibits properties akin to halogens as it can form covalent bonds and diatomic molecules.
Understanding hydrogen's properties and behavior is key not only in chemistry but also in various industrial applications, further emphasizing its significance in both life and the landscape of scientific inquiry.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Basics of Hydrogen
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Hydrogen (H) is the first and lightest element in the periodic table.
● Atomic number: 1
● Atomic mass: 1.008 u
● Exists as a diatomic molecule (H₂) in nature.
Detailed Explanation
Hydrogen is the simplest and lightest element in the periodic table, which means it has an atomic number of 1. The atomic number signifies the number of protons in the nucleus of an atom. Hydrogen's atomic mass is approximately 1.008 atomic mass units (u), indicating its very light weight compared to other elements. In nature, hydrogen commonly exists as a diatomic molecule, represented as H₂, meaning two hydrogen atoms bond together to form a stable molecule.
Examples & Analogies
Think of hydrogen like a single balloon filled with air. If you take two balloons and tie them together, you create a larger, more stable structure. Similarly, two hydrogen atoms bond to form H₂, which is a common form of hydrogen found in nature.
Atomic Structure of Hydrogen
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Atomic number: 1
● Atomic mass: 1.008 u
Detailed Explanation
The atomic number of hydrogen is 1, meaning it contains one proton in its nucleus. It does not have any neutrons in its most common form, making it very light. The atomic mass of 1.008 u shows us how hydrogen atoms weigh slightly more than just one atomic mass unit due to the presence of the electron's mass and other factors. This information is essential for understanding how hydrogen interacts with other elements.
Examples & Analogies
Imagine hydrogen as a single Lego block. The atomic number is like saying you have one block (one proton), while the atomic mass is telling you that this block has a little bit of extra weight, almost like saying it's slightly bigger but still very light compared to other Lego shapes.
Chemical Form of Hydrogen
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Exists as a diatomic molecule (H₂) in nature.
Detailed Explanation
In nature, hydrogen typically does not exist as individual atoms; instead, it pairs up to form molecules, specifically diatomic molecules represented as H₂. This means that two hydrogen atoms bond together, which provides stability and is essential for various chemical reactions, such as combustion and organic processes.
Examples & Analogies
Consider hydrogen like two friends who always stick together. Just like it's common for friends to pair up for activities, hydrogen atoms prefer to join forces and form pairs (H₂) for stability. This teamwork is crucial when hydrogen combines with other elements during chemical reactions.
Key Concepts
-
Diatomic Nature: Hydrogen primarily exists as H₂.
-
Abundance: Hydrogen is the most abundant element in the universe.
-
Position: Unique placement linking properties of both alkali metals and halogens.
-
Physical Properties: Colorless, odorless gas; burns with a pale blue flame.
Examples & Applications
Hydrogen is found in water (H₂O), emphasizing its role in supporting life.
In stars, hydrogen undergoes fusion to produce energy, showcasing its importance in the universe.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hydrogen's light, it flies up high, in H₂ form, it’s not shy.
Stories
Once upon a time, in the vast universe, a particle named Hydrogen danced with Oxygen, forming water droplets that nurtured life on Earth.
Memory Tools
H₂ - Remember: 'Two Happy atoms together!'
Acronyms
H.E.L.P
Hydrogen’s Enacted Life Power – it’s essential to all organic life.
Flash Cards
Glossary
- Hydrogen
The first element in the periodic table, symbol H, characterized by the atomic number 1.
- Diatomic molecule
A molecule that consists of two atoms of the same or different elements, such as H₂.
- Atomic number
The number of protons in the nucleus of an atom; for hydrogen, this is 1.
- Valence electron
An electron in the outer shell of an atom that can be involved in forming chemical bonds.
- Covalent bond
A chemical bond formed by the sharing of electrons between atoms.
Reference links
Supplementary resources to enhance your learning experience.
