Position in the Periodic Table
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Hydrogen's Unique Position
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we are going to explore hydrogen's unique position in the periodic table. Who can tell me what makes hydrogen special compared to other elements?

Is it because it's the first element?

Exactly! Hydrogen is the very first element with atomic number 1. This unique position allows it to display properties similar to both alkali metals and halogens. Let's think of hydrogen's valence electron. How many does it have?

One valence electron!

Correct! This property allows hydrogen to form +1 ions like the alkali metals. Remember our mnemonic 'H for Hybrid'? It means hydrogen shows a mix of properties.

And what about the halogens?

Good question! Like halogens, hydrogen can form covalent bonds and exists naturally as H₂. So what do we call molecules that consist of two atoms?

Diatomic molecules!

Spot on! In summary, hydrogen has one valence electron, forms +1 ions, and can exist as diatomic molecules. This makes it versatile. Keep in mind that remembering 'H for Hybrid' can help you recall its dual properties.
Comparison to Alkali Metals and Halogens
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's compare hydrogen specifically to alkali metals and halogens. Can anyone remind me why hydrogen is similar to alkali metals?

Because it can form +1 ions?

That's right! Alkali metals have one electron that they usually lose easily. Now, how about its resemblance to halogens?

It can form covalent bonds?

Exactly! Both hydrogen and halogens can form diatomic molecules. If we recall that, we could think of the phrase 'HCl is Halogen-like' to remember similar behaviors.

Does this mean hydrogen is sort of a bridge between these two groups?

That’s a great way to think about it! It shares features with both groups, giving it a unique position in chemistry. To sum up, hydrogen’s one electron and its ability to bond covalently link it with both alkali metals and halogens.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In the periodic table, hydrogen is uniquely placed, showing properties of both Group 1 (alkali metals) and Group 17 (halogens). It has characteristics similar to alkali metals due to having one valence electron and the ability to form +1 ions, while also resembling halogens by forming covalent bonds and diatomic molecules.
Detailed
Position in the Periodic Table
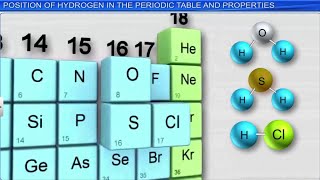
Hydrogen holds a unique position in the periodic table as the first element and is positioned at the top of Group 1, which typically contains alkali metals, and exhibits some properties that are similar to Group 17, known as the halogens.
- Group 1 (Alkali Metals): Hydrogen has 1 valence electron, which allows it to form +1 ions (H⁺). This characteristic is similar to alkali metals that also have one electron in their outermost shell.
- Group 17 (Halogens): Like halogens, hydrogen can form covalent bonds, particularly when it pairs with another hydrogen atom to create diatomic molecules (H₂). Diatomic molecules are fundamental forms of elements that exist naturally in pairs.
This dual nature of hydrogen underscores its versatility and importance in various chemical reactions and its essential role in both organic and inorganic chemistry.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Unique Placement in the Periodic Table
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Unique placement: Shows properties of both Group 1 (alkali metals) and Group 17 (halogens).
Detailed Explanation
Hydrogen has a unique position in the periodic table. It is placed at the top of Group 1 but has properties that are similar to both alkali metals (Group 1) and halogens (Group 17). This means that hydrogen cannot be classified straightforwardly; it exhibits behavior characteristic of both groups.
Examples & Analogies
Think of hydrogen like a student who is very versatile and excels in both science and art. Just as this student has strengths that span different subjects, hydrogen shares characteristics with elements from two distinct groups in the periodic table.
Similarity to Alkali Metals
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Like alkali metals: Has 1 valence electron and forms +1 ions.
Detailed Explanation
Similar to alkali metals, which also have one electron in their outermost shell (valence shell), hydrogen has one valence electron. This allows hydrogen to easily lose this electron, forming a positive ion with a +1 charge (H⁺), much like alkali metals do.
Examples & Analogies
Imagine you have one cookie to share. It's easy for you to give away that single cookie, just like hydrogen easily loses its one outer electron to form an ion. In the same way, alkali metals lose their single electron to achieve stability.
Similarity to Halogens
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Like halogens: Forms covalent bonds and diatomic molecules.
Detailed Explanation
Hydrogen exhibits properties similar to halogens as well. It can form covalent bonds, meaning it can share electrons with other non-metal atoms to create stable molecules. In nature, hydrogen commonly exists as a diatomic molecule (H₂), meaning two hydrogen atoms bond together.
Examples & Analogies
You can think of hydrogen as being like a pair of friends who like to stick together. Just as friends often team up to face challenges, hydrogen atoms join in pairs to create a stable diatomic molecule (H₂) when they are not interacting with other elements.
Key Concepts
-
Unique Position: Hydrogen is positioned in the periodic table where it reflects both alkali metals and halogens.
-
Valence Electron: It has one valence electron, allowing for +1 ion formation.
-
Covalent Bonds: Hydrogen can form diatomic molecules and covalent bonds.
Examples & Applications
Hydrogen is often compared to lithium (Li) from Group 1 and chlorine (Cl) from Group 17.
When hydrogen pairs with another hydrogen atom, it creates H₂, a diatomic molecule.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hydrogen, the first one, one valence, it’s fun!
Stories
Once there was a curious atom named Hydrogen who loved to bond. It met Carbon and formed a chain, and with itself, it danced in pairs, creating H₂, delightful and rare.
Memory Tools
Remember 'H for Hybrid' to recall hydrogen's unique mixture of properties.
Acronyms
H.A.C. - Hydrogen Abundance connects to its Alkali metal and Covalent bonding nature.
Flash Cards
Glossary
- Valence Electron
An electron in the outer shell of an atom, which can be involved in forming bonds.
- Diatomic Molecule
A molecule composed of two atoms, which can be of the same or different chemical elements.
- Ionic Bond
A type of chemical bond that involves the electrostatic attraction between oppositely charged ions.
- Covalent Bond
A type of bond where two atoms share one or more pairs of electrons.
Reference links
Supplementary resources to enhance your learning experience.
