Applications of Gas Laws
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Explaining Behavior of Gases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore how gas laws help us understand everyday phenomena, like the behavior of gases in balloons and syringes. Can anyone explain how gas pressure changes when a balloon is inflated?

As I blow into the balloon, the pressure inside increases, right?

Exactly! This is a perfect example of Boyle’s Law, where volume and pressure inversely relate. Remember, more air means more pressure inside, but it can only stretch so far! What happens if we keep inflating it?

It might pop when the pressure is too high!

Correct! Knowing this helps us understand how to handle gases safely. Remember: high pressure can cause failure; that's a key point!
Industrial Applications of Gas Laws
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s delve into how gas laws apply in industrial applications. Can someone provide an example of how industries utilize these laws?

Gas storage in tanks? They have to manage pressure carefully.

Exactly! It’s crucial. Industries often need to maintain specific pressures and volumes to ensure safety and efficiency. Let’s think about what might happen if a tank isn’t designed according to these laws.

It could explode or leak!

Right! That's why understanding gas behavior is fundamental in these environments. Remember the acronym 'PAVE' - Pressure, Apply, Validate, Ensure - to keep safe in industrial contexts.
Weather Balloons and Air Pressure Devices
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s discuss weather balloons. Who knows how they work in relation to gas laws?

They expand as they rise, right? Because the pressure decreases?

That's right! As they ascend, the atmospheric pressure drops, allowing the balloon to expand. This behavior helps scientists gather crucial weather data. Can anyone tell me how air pressure devices like barometers relate?

They measure changes in air pressure, which influences the weather!

Exactly! By understanding these principles, we can predict weather patterns. Remember - when air pressure changes, weather changes!
Significance in Chemical Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s look at the significance of gas laws in chemical reactions. How do we use these concepts when dealing with gases in reactions?

We need to know the volume and temperature to predict reaction outcomes.

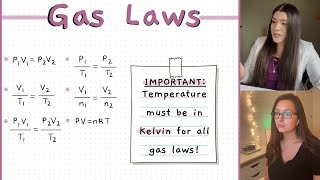
Yes! For instance, during gas reactions, observing changes in volume helps us understand the reaction's extent. Can someone remind us why we convert temperature to Kelvin in these calculations?

So that we can maintain the accuracy needed for gas law calculations?

Exactly! Precision is vital in chemistry. Let's summarize: gas laws help explain behavior in various real-life applications, ensuring we do it safely and efficiently!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The applications of gas laws extend to multiple contexts, from explaining the behavior of gases in everyday objects like balloons and syringes to their significance in industrial practices and weather-related technologies. Understanding these applications is crucial for comprehending various scientific and practical phenomena involving gases.
Detailed
Applications of Gas Laws
Gas laws provide us with insights on how gases behave under different physical conditions. Understanding these applications is essential not just for academic knowledge but also for real-world problem-solving. Here are the major applications:
- Explaining Behavior of Gases: Gas laws can elucidate the behavior of gases in everyday tools such as balloons and syringes. For example, when you pull the plunger of a syringe, you create a low-pressure area that draws liquid into the syringe, demonstrating Boyle's Law.
- Industrial Applications: In industries, gas laws are critical for processes involving gas storage and pressurization. Storage tanks must adhere to these laws to ensure that gases are maintained at safe pressures and volumes.
- Weather Balloons: Weather balloons utilize gas laws as they expand or contract with changes in temperature and atmospheric pressure, aiding in meteorological data collection.
- Air Pressure Devices: Devices such as barometers rely on gas law principles to measure environmental pressure, which is crucial for weather forecasting and studying environmental phenomena.
- Chemical Reactions: Understanding gas behavior is fundamental when analyzing chemical equations that involve gaseous reactants or products, allowing predictions about reaction conditions and outcomes.
By applying gas laws to these domains, we gain a deeper appreciation for the principles governing our surroundings.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Behavior of Gases in Everyday Objects
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Explaining behavior of gases in balloons, syringes, and scuba diving
Detailed Explanation
This chunk discusses how gas laws explain the behavior of gases in everyday objects. For example, when you inflate a balloon, the volume of the gas inside increases while the pressure inside decreases. This is a practical application of Boyle's Law, which states that at constant temperature, the volume of a fixed mass of gas is inversely proportional to its pressure. Similarly, when using a syringe, pulling the plunger creates a vacuum that allows gas to fill the space, demonstrating gas behavior and concepts such as pressure and volume relationships.
Examples & Analogies
Consider a balloon: when you blow air into it, the volume of the balloon expands. This is like a car tire being filled with air; the more air you pump in, the bigger and firmer it gets, demonstrating how gas expands to fill available space.
Industrial Applications
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Industrial applications (e.g., gas storage, pressurization)
Detailed Explanation
In industries, understanding gas laws is crucial for processes involving gas storage and pressurization. Gas laws help engineers design equipment like gas cylinders and pipelines that maintain the appropriate pressure levels to prevent explosions or leaks. For example, in natural gas storage facilities, the volume and pressure of gas must be precisely controlled to ensure safety and efficiency.
Examples & Analogies
Think of a soda can: when the can is sealed, carbon dioxide gas is under high pressure. Opening the can releases this pressure, allowing the gas to escape and the liquid to expand, which illustrates how gas laws are not only theoretical but also essential in creating safe beverage containers.
Weather Balloons and Air Pressure Devices
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Weather balloons and air pressure devices
Detailed Explanation
Gas laws apply to meteorology through the use of weather balloons. These balloons rise in the atmosphere, and as they ascend, the pressure decreases which allows the gas inside to expand. Scientists use these changes to gather data about atmospheric pressure and temperature. Similarly, devices that measure air pressure, like barometers, rely on the principles of gas laws to function correctly.
Examples & Analogies
Imagine a weather balloon rising into the sky: as it goes higher, the outer pressure decreases and makes the balloon expand, just like how a balloon filled with helium rises. This helps meteorologists predict the weather, much like how a weather report gives us clues about our day!
Understanding Chemical Equations Involving Gases
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Fundamental to understanding chemical equations involving gases
Detailed Explanation
Gas laws are essential for interpreting chemical equations that involve gaseous reactants or products. For instance, when gas is produced in a reaction, understanding the relationships between pressure, volume, and temperature helps in calculating how much gas will be generated. This knowledge is vital in fields such as chemistry, engineering, and environmental science.
Examples & Analogies
Consider baking bread: when yeast ferments, it produces carbon dioxide gas. Knowledge of gas laws helps bakers understand how much gas will form, which in turn inflates the bread. Thus, the chemistry behind baking involves gas laws just as much as it does the ingredients!
Key Concepts
-
Applications of gas laws in daily life: Gas laws help explain the behavior of gases in items like balloons and syringes.
-
Industrial importance: Industries rely on understanding gas laws for safe and efficient gas storage and management.
-
Meteorological significance: Weather balloons utilize gas laws to collect atmospheric data.
-
Chemical reaction predictions: Gas laws are fundamental in predicting outcomes involving gases in chemical reactions.
Examples & Applications
Inflation of a balloon demonstrates Boyle's Law as pressure inside increases and volume changes.
Gas pressure management in industrial tanks to prevent explosions or leaks.
Weather balloons expand with rising altitude due to decreasing atmospheric pressure.
Barometers measure atmospheric pressure to predict weather changes.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the skies a balloon does rise, expanding far as pressure flies.
Stories
Once upon a time, a brave weather balloon set out to gather information, rising high as it expanded under the soft whispers of air pressure – it loved how gas laws guided its adventure!
Memory Tools
Remember 'B.I.G' for applications of gas laws: Balloons, Industrial processes, and Gases in reactions.
Acronyms
PAVE for gas safety
Pressure
Apply
Validate
Ensure.
Flash Cards
Glossary
- Gas Laws
A set of laws that describe the behavior of gases in relation to pressure, volume, and temperature.
- Boyle’s Law
States that at constant temperature, the volume of a fixed mass of gas is inversely proportional to its pressure.
- Charles’s Law
States that at constant pressure, the volume of a fixed mass of gas is directly proportional to its absolute temperature.
- Combined Gas Law
Combines Boyle’s and Charles's Laws into a single equation that relates pressure, volume, and temperature.
- STP
Standard Temperature and Pressure, defined as 0°C (273 K) and 1 atm (760 mmHg).
Reference links
Supplementary resources to enhance your learning experience.
