Is there a difference in the concentrations of ions?
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ions and Their Concentrations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore ions in acids and bases. Can anyone tell me what ions are?

Ions are charged particles.

Correct! Acids produce hydrogen ions, which are positively charged. Can you remember the ion that bases produce?

Yes, they produce hydroxide ions.

Right again! Now, what do you think happens when we mix an acid with a base?

They neutralize each other!

Exactly! Remember, H+ from acids and OH- from bases react to form water.

Let’s remember that H+ is the key character of acids. You can recall it by the mnemonic 'H is for Hydrogen in acids'.

That's helpful!
Indicators and Ion Detection
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, who can tell me what an indicator is?

An indicator is a substance that changes color based on pH.

Correct! Indicators help us detect the presence of H+ or OH- ions. Can you name a common indicator?

Litmus paper!

Excellent! Litmus paper turns red in acidic solutions. Who remembers the color change for bases?

It turns blue!

Exactly! Think of the acronym ‘RAB’—Red for Acid, Blue for Base. Always keep an eye on those colors when testing.

That's a great way to remember!
Conductivity and Ion Concentration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s talk about how acids and bases conduct electricity. Why do you think this is?

Because they contain ions!

Exactly! More ions mean better conductivity. Can you think of an experiment we can do to test this?

We could use a simple circuit with a bulb and see if it lights up with different solutions!

Perfect! By testing various solutions, we can observe that strong acids will light the bulb brighter than weak acids due to higher ion concentration. Remember the phrase 'More ions, more light!'

That will help me remember.
Experimental Observations of Ion Concentration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s reflect on our experimental results using indicators. What was our initial hypothesis?

I think we expected different colors for acids and bases.

Exactly! And did our observations match our predictions?

Yes, when we added acidic solutions to the indicator, it changed to red.

Great! This direct observation reinforces our understanding of how concentration affects acidity. Remember the key concept: 'Color change indicates ion concentration!'

That’s very clear now!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section elaborates on how concentrations of ions differ in acid and base solutions, explores how this impacts their reactivity, and provides insights into testing methodologies using indicators to identify acids and bases.
Detailed
In this section, we delve into the differences in the concentrations of ions present in acidic and basic solutions. The understanding of ion concentrations is crucial since they dictate the chemical behavior of these substances. Acids produce hydrogen ions (H+) when dissolved in water, contributing to their acidic properties. Conversely, bases produce hydroxide ions (OH-). This leads to the necessity of using indicators, which are substances that change color in response to pH levels, to identify whether a solution is acidic or basic. The section also includes practical experiments demonstrating how to test solutions and interpret the results based on the observed colors, thus solidifying the connection between ion concentration and chemical properties.
Youtube Videos





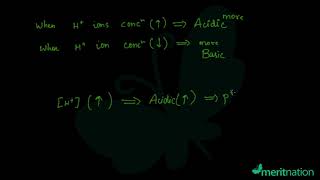

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Ions in Acids and Bases
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In Section 2.1, we have seen that all acids have similar chemical properties. What leads to this similarity in properties? We saw in Activity 2.3 that all acids generate hydrogen gas on reacting with metals, so hydrogen seems to be common to all acids.
Detailed Explanation
This chunk discusses how all acids exhibit similar behaviors due to a common element: the hydrogen ion (H+). When acids react with metals, they produce hydrogen gas, confirming the presence of H+. The key takeaway is that the defining characteristic of acidic behavior in aqueous solutions is the release of hydrogen ions.
Examples & Analogies
Think of hydrogen ions as tiny 'signals' that tell us a solution is acidic, much like a sports referee who blows a whistle to signal a foul. Wherever you see that whistle blown (the presence of H+), you know the game (the chemical reaction) involves an acid.
Investigating Ion Production in Solutions
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let us perform an Activity to investigate whether all compounds containing hydrogen are acidic.
Detailed Explanation
This activity prompts students to test various solutions containing hydrogen, such as glucose and alcohol, and observe whether they can conduct electricity. The ability to conduct electricity suggests that a solution contains ions, and they are looking for the presence of H+ ions specifically. If the bulb lights up, it indicates the solution is acidic due to the presence of H+ ions.
Examples & Analogies
Imagine a light bulb that represents knowledge. When hydrogen ions are present in a solution, it's like the light bulb gets turned on. You can only turn on the bulb (show that the solution is acidic) if you have the right key (hydrogen ions). In contrast, substances like glucose or alcohol lack this key, so the bulb stays off.
The Role of Water in Ion Formation
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Do acids produce ions only in aqueous solution?
Detailed Explanation
In this part, the text explains that acids, such as hydrochloric acid (HCl), can only generate hydrogen ions (H+) in the presence of water. This action shows that without water, the ionization that leads to acidity doesn't occur. Therefore, when acids are dissolved in water, they dissociate into ions, the key factor for their acidic behavior.
Examples & Analogies
Think of water as a stage for a play where the actors (hedgehogs, in this case, are hydrogen ions) perform. Without the stage (water), the actors cannot act. When you add water, they can come out and interact. This is how acids behave—only in aqueous solutions can they reveal their true nature by producing H+ ions.
Dilution and Concentration of Ions
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Mixing an acid or base with water results in a decrease in the concentration of ions (H+(aq)/OH–(aq) per unit volume. Such a process is called dilution and the acid or the base is said to be diluted.
Detailed Explanation
Here, the text highlights the concept of dilution, which occurs when an acid or base is mixed with water, resulting in a lower concentration of hydrogen or hydroxide ions per unit volume. This concept is crucial because it explains how the strength of an acid or a base is affected by the amount of water mixed in.
Examples & Analogies
Imagine pouring a little juice concentrate into a pitcher and then adding water to it. The more water you add, the less intense the flavor becomes. Similarly, diluting an acid or base with water reduces the concentration of its ions, making it 'weaker' in terms of acidity or alkalinity.
pH and the Strength of Acids and Bases
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
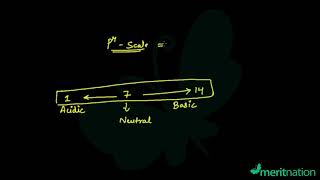
The pH of a neutral solution is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, it represents an increase in OH– ion concentration in the solution, that is, increase in the strength of alkali.
Detailed Explanation
This chunk explains the pH scale as a means to measure the strength of acids and bases. A pH of 7 is neutral, less than 7 indicates an acid, and greater than 7 indicates a base. The key connection here is that the pH reflects the balance of H+ and OH– ions in a solution. The scale helps students to quantify acidic and basic properties.
Examples & Analogies
Think of the pH scale like a balance scale used in cooking. A recipe might require precise measurements; too much salt (acid) or too much sugar (base) can spoil the dish (solution). The pH scale helps us 'measure' how much of each we have, ensuring the right balance for the perfect dish!
Key Concepts
-
Acids produce hydrogen ions (H+) when dissolved in water.
-
Bases produce hydroxide ions (OH-) when dissolved in water.
-
Indicators help identify acids and bases based on color changes.
-
Higher ion concentration in a solution affects its conductivity.
Examples & Applications
Lemon juice is acidic and changes litmus paper to red due to high H+ concentration.
Baking soda solution is basic and changes litmus paper to blue due to high OH- concentration.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In an acid, H+ you see, in a base, OH- will be!
Stories
Once in a lab, the acid met the base. A dance of H+ and OH- took place when they embraced!
Memory Tools
Remember: 'A is for Acids producing H+, B is for Bases producing OH-.'
Acronyms
Use 'A&L' for acids and litmus, as they are linked in testing acidity!
Flash Cards
Glossary
- Ion
A charged particle that forms when an atom or molecule gains or loses one or more electrons.
- Hydrogen ion (H+)
A positively charged ion formed from an acid in solution.
- Hydroxide ion (OH)
A negatively charged ion formed from a base in solution.
- Indicator
A substance that changes color when added to an acid or a base.
- pH
A measure of the acidity or basicity of a solution, where lower values are more acidic and higher values are more basic.
Reference links
Supplementary resources to enhance your learning experience.
