Reaction of Metallic Oxides with Acids
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Metallic Oxides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to discuss how metallic oxides react with acids. Can anyone tell me what a metallic oxide is?

A metallic oxide is a chemical compound made from a metal and oxygen!

Exactly! So, when a metallic oxide reacts with an acid, what do you think happens?

Do they form salts and water?

Yes, that's correct! The general reaction is: Metal oxide + Acid → Salt + Water. To remember this, think 'MAWS'—Metallic Oxide + Acid = Water and Salt.

What does 'water and salt' mean in this context?

Good question! It means we create a salt, which is the result of the acid and the metallic oxide reacting together and water. Let's look at an example.
Example Reaction: Copper Oxide and Hydrochloric Acid
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

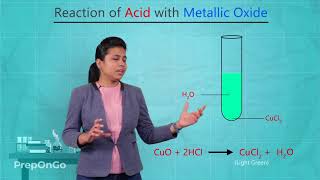
Let’s take copper oxide and hydrochloric acid as an example. What do you think will happen when we mix them?

Will it dissolve and turn blue?

Yes! When you add hydrochloric acid to copper oxide, the copper oxide dissolves and you get a blue-green solution of copper(II) chloride.

Why does it turn blue-green?

That's because copper(II) chloride is blue-green. It's a nice visual example of how we can see the reaction happening!

So, does that mean copper oxide is a basic oxide?

Exactly! Because it reacts like a base with an acid. Remember that metallic oxides are indeed basic oxides.
Balancing Chemical Equations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about how to write and balance the equation for the reaction we just discussed.

What is the equation for the copper oxide and hydrochloric acid reaction?

"The reaction can be shown as:
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the reaction of metallic oxides, which are basic in nature, with acids. The key outcome of this reaction is the formation of salts and water, reinforcing the concept that metallic oxides behave like bases.
Detailed
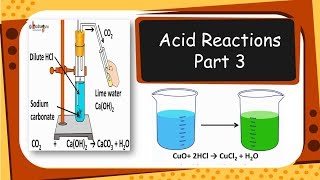
In the reaction between metallic oxides and acids, metallic oxides, such as copper oxide, combine with acidic solutions, resulting in the production of salts and water. For example, when copper oxide reacts with hydrochloric acid, it dissolves to form copper(II) chloride, which is a blue-green solution. The general reaction can be summarized as:
Metal oxide + Acid → Salt + Water.
This concept reinforces that metallic oxides are basic oxides and exhibit behaviors similar to those of bases in their reactions with acids. Understanding these reactions is fundamental in comprehending the broader principles of acid-base interactions.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to the Reaction
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Take a small amount of copper oxide in a beaker and add dilute hydrochloric acid slowly while stirring.
Detailed Explanation
In this step, we are starting an experiment with copper oxide and hydrochloric acid. Copper oxide is a type of metallic oxide. When we add the acid to the copper oxide, it's important to do so slowly and stir the mixture to allow the reaction to occur properly. The method involves observing how the materials mix together and what changes happen during this process.
Examples & Analogies
Think of it like making a smoothie. You add fruit (copper oxide) to milk (hydrochloric acid) slowly and blend (stir) it. You notice how the mixture changes color and texture as you combine them.
Observation of Color Change
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Note the colour of the solution. What has happened to the copper oxide?
Detailed Explanation
As you mix the copper oxide with hydrochloric acid, you should observe that the color of the solution changes to blue-green. This color indicates that a reaction is taking place, resulting in the formation of a new compound, copper(II) chloride, dissolved in the solution. The copper oxide actually dissolves in the acid, transforming the initial solid into a liquid.
Examples & Analogies
Imagine adding sugar (copper oxide) to water (hydrochloric acid). At first, the sugar is solid and doesn't mix in. But as you stir, it dissolves and changes the appearance of the water. This is similar to what happens in this chemical reaction.
General Reaction Equation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The general reaction between a metal oxide and an acid can be written as –
Metal oxide + Acid → Salt + Water
Detailed Explanation
This equation summarizes what happens in the reaction between metal oxides and acids. In this case, copper oxide (the metal oxide) reacts with hydrochloric acid to produce copper(II) chloride (the salt) and water. The concept of acids reacting with metal oxides is similar to how bases react with acids. Metal oxides are classified as basic oxides because they exhibit behavior similar to that of bases in their reactions with acids.
Examples & Analogies
Consider baking soda (a base) reacting with vinegar (an acid) to produce carbon dioxide gas, water, and a salt. Just like that, metal oxides also undergo a reaction with acids, producing a salt and water.
Classification of Metallic Oxides
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Since metallic oxides react with acids to give salts and water, similar to the reaction of a base with an acid, metallic oxides are said to be basic oxides.
Detailed Explanation
Metallic oxides are categorized as basic oxides because they follow the same principles as bases when they react with acids. Their capacity to neutralize acids and produce salt and water during reactions confirms their basic nature. This is a critical feature for understanding how different compounds behave chemically.
Examples & Analogies
Imagine how an antacid (acting as a base) neutralizes stomach acid for relief. Basic oxides do this in a similar way when they interact with acids, helping us understand the broader category of acids and bases.
Key Concepts
-
Metallic Oxides: Compounds formed by a metal and oxygen that can react with acids.
-
Reaction with Acids: Metallic oxides react with acids to form salts and water.
-
Basic Oxides: Metallic oxides are considered basic due to their ability to neutralize acids.
Examples & Applications
Copper oxide reacting with hydrochloric acid produces copper(II) chloride and water.
Zinc oxide reacting with sulfuric acid produces zinc sulfate and water.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Metal oxide and acid mix, to create salts, a handy fix!
Stories
Imagine a colorful potion turning blue-green. That's copper oxide meeting hydrochloric acid, producing something new!
Memory Tools
Think 'MAWS': Metal Oxide + Acid = Water and Salt.
Acronyms
MOS
Metal Oxides are Salts when they react with acids.
Flash Cards
Glossary
- Metallic Oxides
Compounds formed by the combination of a metal and oxygen, often basic in nature.
- Salts
Ionic compounds formed from the neutralization reaction between an acid and a base.
- Hydrochloric Acid
A strong acid commonly used in reactions with metals and oxides.
- Copper(II) Chloride
A blue-green salt formed when copper oxide reacts with hydrochloric acid.
Reference links
Supplementary resources to enhance your learning experience.
