Chemical Reactions and Equations
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Chemical Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to discuss chemical reactions! Can anyone tell me what a chemical reaction is?

Is it when substances change into new substances?

Exactly! When substances undergo a change in their identity, that’s a chemical reaction. For example, when magnesium burns in air, it forms magnesium oxide.

What signs tell us that a chemical reaction has occurred?

Good question! We can observe changes in color, temperature, gas evolution, or state of matter. Let’s remember the acronym CTEG: Color, Temperature, Evolved gas, and Change of state.

Can we see an example of this?

Yes! In our activity, burning a magnesium ribbon is a perfect example. At the end of our session, you'll see it change dramatically!

Let's recap what we've learned: chemical reactions involve changes that can be observed, and we have our CTEG acronym to remember the indicators!
Types of Chemical Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know what reactions are, let’s dive deeper. Can anyone name a type of chemical reaction?

Combination reactions?

That’s right! In a combination reaction, two or more reactants combine to form a single product. Can anyone give an example?

Like when calcium oxide reacts with water to form slaked lime?

Perfect! Now, what about decomposition reactions?

That’s when a single reactant breaks down into multiple products, right?

Exactly! Consider the decomposition of ferrous sulfate upon heating. Remember: COFFEE for Combination, Oxidation, Decomposition, and Double displacement. Keeping our key types of reactions organized will help us in our studies!
Balancing Chemical Equations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s talk about balancing chemical equations. Why do you think it is important to balance them?

So that we follow the law of conservation of mass?

Exactly! The number of atoms for each element must be the same on both sides of the equation. Can anyone tell me how we check this?

We count the atoms in the reactants and products.

Yes! Here’s a trick: I like to use the term 'Tally' - like making tallies for each element to ensure we account for every atom. Now, let’s practice with an equation. How would we balance the reaction of iron and water?

We would list the number of each atom on both sides and adjust coefficients accordingly.

Brilliant! Now, let’s wrap up with our acronym 'TALLY' to remember the balancing process!
Oxidation and Reduction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s explore oxidation and reduction. Who knows what happens during oxidation?

Is it when a substance gains oxygen?

Correct! And what about reduction?

It’s when a substance loses oxygen?

Exactly! Ans we can remember Oxygen Award: Oxidation is gaining oxygen while Reduction is losing it. Can we think of real-life examples?

Rusting is oxidation, right?

Yes, rusting is a common example. Let’s summarize our notes with the 'Award' role play, where we'll assign oxidation and reduction roles to different elements!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explores various types of chemical reactions, including combination, decomposition, displacement, and double displacement. It emphasizes the importance of balancing chemical equations and understanding physical states. Activities and examples are used to illustrate the changes that indicate chemical reactions.
Detailed
Chemical Reactions and Equations
This section covers the fundamental concepts of chemical reactions and how they are represented through equations. A chemical reaction occurs when substances undergo a process leading to the transformation of their identities. The initial substances, known as reactants, transform into new substances called products. The section highlights the indications of a chemical reaction, such as changes in state, color, temperature, and gas evolution.
Key Types of Chemical Reactions
- Combination Reactions: Two or more substances combine to form a single product, exemplified by the reaction of calcium oxide with water to yield slaked lime.
- Decomposition Reactions: A single compound breaks down into two or more products, such as the thermal decomposition of ferrous sulfate into iron oxide and sulfur oxides.
- Displacement Reactions: One element displaces another in a compound, as seen when iron displaces copper from copper sulfate solution.
- Double Displacement Reactions: Ions exchange between two compounds to form new compounds, such as when barium chloride reacts with sodium sulfate.
Additionally, the importance of balancing chemical equations is emphasized, as it reflects the law of conservation of mass. The section also discusses oxidation and reduction reactions, where substances gain or lose oxygen. Lastly, the lesson covers concepts like corrosion and rancidity, highlighting their relevance in everyday life.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Examples of Chemical Changes
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consider the following situations of daily life and think what happens when –
- milk is left at room temperature during summers.
- an iron tawa/pan/nail is left exposed to humid atmosphere.
- grapes get fermented.
- food is cooked.
- food gets digested in our body.
- we respire.
In all the above situations, the nature and the identity of the initial substance have somewhat changed. We have already learnt about physical and chemical changes of matter in our previous classes. Whenever a chemical change occurs, we can say that a chemical reaction has taken place.
Detailed Explanation
This chunk introduces various scenarios from everyday life that illustrate chemical changes. In each case, the original substances (like milk, iron, grapes, and food) undergo transformations that alter their identities. For example, when food is cooked, its chemical composition changes, leading to different flavors and textures. Chemical changes are distinct from physical changes, where the substance's state may change (like ice melting) but its chemical nature does not.
Examples & Analogies
Think of making pancakes. When you mix flour and eggs (original substances), and then cook the mixture (chemical change), it transforms into a completely different substance—pancakes! You can no longer separate the flour and eggs as they were.
Understanding Chemical Reactions
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
You may perhaps be wondering as to what is actually meant by a chemical reaction. How do we come to know that a chemical reaction has taken place? Let us perform some activities to find the answer to these questions.
Detailed Explanation
This chunk invites students to consider what a chemical reaction entails. A chemical reaction involves making and breaking bonds between atoms, leading to the transformation of products. The text suggests performing activities to observe chemical reactions and their indicators, such as color changes, gas production, and temperature changes.
Examples & Analogies
Imagine baking bread. The dough rises as yeast ferments sugars—this is a chemical reaction. You notice the dough expanding (a visual indicator of a reaction taking place).
Observations from Activities
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
From the above three activities, we can say that any of the following observations helps us to determine whether a chemical reaction has taken place –
- change in state
- change in colour
- evolution of a gas
- change in temperature.
Detailed Explanation
This chunk summarizes the key indicators that signify a chemical reaction has occurred. Changes in state (solid to liquid), color (blue to yellow), gas evolution (bubbles during reactions), or temperature fluctuations (warmth from an exothermic reaction) are clear signs of chemical changes. Observing these changes helps scientists confirm whether a reaction has taken place.
Examples & Analogies
Consider mixing baking soda and vinegar. You see bubbling (gas evolution), hear fizzing sounds, and feel a temperature drop (change in temperature)—all indicators of a chemical reaction happening right before your eyes!
Writing Word Equations
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The word-equation for the above reaction would be –
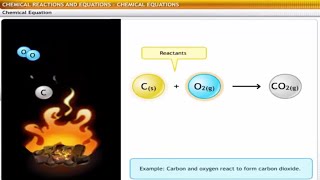
Magnesium + Oxygen → Magnesium oxide (Reactants) (Product)
Detailed Explanation
This chunk introduces how to represent chemical reactions using word equations. Reactants are listed on the left side, products on the right, with an arrow indicating the transformation. This format, though verbal, simplifies understanding the components involved in a reaction.
Examples & Analogies
Think of a recipe. Just as a recipe lists the ingredients and the dish produced, a word equation outlines the reactants (ingredients) and product (final dish) in a chemical reaction.
Using Chemical Formulae
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A chemical equation represents a chemical reaction. If you recall formulae of magnesium, oxygen and magnesium oxide, the above word-equation can be written as –
Mg + O → MgO.
Detailed Explanation
This chunk explains the transition from word equations to chemical formulae, which provide a more concise representation of reactions. Scientific symbols simplify communication and calculations in chemistry, ensuring clarity in describing complex reactions.
Examples & Analogies
Imagine shorthand notes. Just as shorthand condenses written language for efficiency, chemical formulae distill the components of a chemical reaction into symbols for quicker understanding and calculations.
Balancing Chemical Equations
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Count and compare the number of atoms of each element on the LHS and RHS of the arrow. If the number of atoms of each element is the same on both sides, the equation is balanced.
Detailed Explanation
This chunk highlights the importance of balancing chemical equations, adhering to the law of conservation of mass. Each side of the equation must have an equal number of atoms for each element to reflect a true chemical reaction without change in mass.
Examples & Analogies
Think of balancing a scale. Just as you would need to add or change weights to make both sides equal, in chemistry, you must adjust coefficients in a reaction equation to ensure both sides have equal elements.
Physical States in Chemical Equations
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To make a chemical equation more informative, the physical states of the reactants and products are mentioned along with their chemical formulae.
Detailed Explanation
This chunk explains that when writing chemical equations, indicating the physical states of substances helps clarify how they exist during reactions. These notations (solid, liquid, gas, aqueous) provide context for how reactions proceed in different environments.
Examples & Analogies
Consider cooking again—if a recipe doesn't mention whether ingredients should be dry, liquid, or frozen, it would be confusing. Similarly, knowing the physical states in a reaction ensures clarity about how substances interact.
Key Concepts
-
Chemical Reaction: A transformation resulting in new substances.
-
Reactants: Initial compounds before the reaction.
-
Products: End results after a reaction occurs.
-
Combination Reaction: Forming one product from multiple reactants.
-
Decomposition Reaction: Breaking down one substance into simpler forms.
-
Displacement Reaction: One element taking the place of another.
-
Double Displacement Reaction: Ion exchange between compounds.
Examples & Applications
Example 1: Burning magnesium ribbon shows a combination reaction.
Example 2: Heating ferrous sulfate resulting in decomposition and formation of new substances.
Example 3: Iron displacing copper in copper sulfate indicating a displacement reaction.
Example 4: Barium chloride reacting with sodium sulfate yielding a precipitate, illustrating double displacement.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a combo, we come together, in a break, we split our tether.
Stories
Once upon a time, a lonely element combined with another, forming a stronger compound; they lived happily as a single entity.
Memory Tools
For remembering reaction types: COFFEE - Combination, Oxidation, Decomposition, and Double displacement.
Acronyms
TALLY for balancing equations
Tally atoms
Adjust coefficients
Look for discrepancies
and yield a balanced equation.
Flash Cards
Glossary
- Chemical Reaction
A process that leads to the transformation of one set of chemical substances to another.
- Reactants
The initial substances that undergo a change in a chemical reaction.
- Products
The new substances formed as a result of a chemical reaction.
- Combination Reaction
A chemical reaction where two or more substances combine to form a single product.
- Decomposition Reaction
A chemical reaction in which a single compound breaks down into two or more simpler substances.
- Displacement Reaction
A reaction where an element displaces another in a compound.
- Double Displacement Reaction
A reaction between two compounds where an exchange of ions takes place.
Reference links
Supplementary resources to enhance your learning experience.
