Oxidation and Reduction
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Oxidation and Reduction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will learn about oxidation and reduction. Can anyone tell me what happens during oxidation?

I think oxidation is when something gains oxygen?

Correct! Oxidation is indeed the gain of oxygen. It can also involve losing electrons. Now, what about reduction?

Is reduction the opposite of oxidation, like losing oxygen?

Exactly! Reduction refers to a substance losing oxygen or gaining hydrogen. Remember this with the acronym 'OIL RIG' which stands for 'Oxidation Is Loss, Reduction Is Gain'.

Can you give some examples of these reactions?

Sure! For example, when copper is heated in the presence of oxygen, it becomes copper(II) oxide. What would that represent?

That's oxidation because oxygen is added.

Well done! Let's summarize: Oxidation involves gaining oxygen or losing hydrogen, while reduction involves losing oxygen or gaining hydrogen.
Redox Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss redox reactions. What do you think that means?

Is it when oxidation and reduction happen at the same time?

Exactly! In a redox reaction, one substance is oxidized while another is reduced. Let's look at the example: ZnO + C → Zn + CO.

In this reaction, what is oxidized and what is reduced?

Zinc oxide is reduced to zinc, while carbon is oxidized to carbon monoxide. Remember, you could categorize reactions based on the elements involved.

So the identifying part is to see who gains or loses oxygen?

Exactly that! Always check who is gaining or losing oxygen. Let's summarize: redox reactions involve simultaneous oxidation and reduction.
Real-Life Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone think of where oxidation and reduction are important in our daily lives?

Rusting of iron, right?

Absolutely! Rusting is an oxidation reaction, where iron reacts with oxygen and moisture to form iron oxide. What else?

What about digestion?

Right again! During respiration, glucose is oxidized to release energy. That's a biological example of redox reactions. Know this helps in understanding processes like energy generation.

So, is it true we should care about oxidation and reduction to prevent rust?

Correct! Preventive measures like painting or galvanization are applied to reduce oxidation and corrosion.

And that helps us maintain metal structures!

Exactly, let's summarize: oxidation and reduction are essential in everything from biology to construction.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Oxidation and reduction are fundamental concepts in chemistry where oxidation refers to the gain of oxygen or loss of hydrogen, while reduction is the opposite. This section includes examples like the oxidation of copper and details on redox reactions, demonstrating how certain substances oxidize and reduce during chemical processes.
Detailed
Oxidation and Reduction
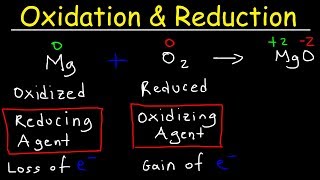
In this section, we delve into the concepts of oxidation and reduction, two vital processes in chemical reactions. Oxidation is characterized by the loss of electrons, typically involving the gain of oxygen. For example, when copper powder is heated in the presence of oxygen, it forms black copper(II) oxide:
2Cu + O → 2CuO. The opposite process, reduction, occurs when a substance loses oxygen or gains hydrogen, as demonstrated when copper(II) oxide is heated with hydrogen, regenerating copper: CuO + H → Cu + H₂O.
This interplay of oxidation and reduction defines redox reactions, where one reactant is oxidized while the other is reduced. Examples include reactions involving zinc oxide and carbon, producing zinc and carbon monoxide (ZnO + C → Zn + CO), and the reduction of manganese oxide by hydrochloric acid (MnO + 4HCl → MnCl + 2H₂O + Cl). Understanding these processes is crucial as they underpin many biochemical and industrial reactions.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Oxidation
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
n Heat a china dish containing about 1 g copper powder (Fig. 1.10).
n What do you observe?
The surface of copper powder becomes coated with black copper(II) oxide. Why has this black substance formed?
This is because oxygen is added to copper and copper oxide is formed.
Detailed Explanation
In this first activity, we heat copper powder. When copper is heated in the presence of oxygen, it reacts and forms copper(II) oxide, which is a black substance. This process of forming copper oxide indicates that copper is undergoing oxidation since it gains oxygen, which is the key criterion for oxidation.
Examples & Analogies
Think of oxidation as a person gaining weight. Just like someone might gain weight by eating more food (analogous to gaining oxygen), copper gains its new property when it reacts with oxygen to become copper(II) oxide.
Understanding Reduction
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If hydrogen gas is passed over this heated material (CuO), the black coating on the surface turns brown as the reverse reaction takes place and copper is obtained.
CuO + H → Cu + H O
Detailed Explanation
In this step, when we pass hydrogen gas over the oxidized copper oxide (CuO), a chemical reaction occurs where copper oxide is reduced back to copper. Here, the oxygen is removed from copper oxide, which is the process of reduction.
Examples & Analogies
Imagine a person losing weight. In this analogy, losing weight is like reducing oxygen from copper oxide, bringing it back to its original state as copper.
Oxidation-Reduction Reactions
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If a substance gains oxygen during a reaction, it is said to be oxidised. If a substance loses oxygen during a reaction, it is said to be reduced. During this reaction (1.29), the copper(II) oxide is losing oxygen and is being reduced. The hydrogen is gaining oxygen and is being oxidised.
Detailed Explanation
During a chemical reaction, whether a substance is oxidised or reduced depends on the movement of oxygen. Gaining oxygen or losing hydrogen indicates oxidation, while losing oxygen or gaining hydrogen indicates reduction. In our example, copper(II) oxide loses oxygen and hydrogen is oxidized by gaining that oxygen.
Examples & Analogies
Consider a light switch: when you turn on the light, you are gaining brightness (which can be likened to gaining oxygen). Conversely, when you turn off the light, the brightness decreases, similar to losing oxygen.
Examples of Redox Reactions
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Some other examples of redox reactions are:
ZnO + C → Zn + CO
MnO + 4HCl → MnCl + 2H O + Cl
Detailed Explanation
In these examples, zinc oxide reacts with carbon to reduce to zinc and carbon monoxide, showing reduction of zinc oxide and oxidation of carbon. Similarly, in the second reaction, manganese oxide reacts with hydrochloric acid showing oxidation of HCl and reduction of MnO.
Examples & Analogies
Think of a seesaw as an analogy for redox reactions. When one side goes up (oxidation), the other side has to go down (reduction). They maintain balance during the reaction, reflecting the interplay of gaining and losing oxygen.
Conclusion of Oxidation-Reduction
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
From the above examples we can say that if a substance gains oxygen or loses hydrogen during a reaction, it is oxidised. If a substance loses oxygen or gains hydrogen during a reaction, it is reduced.
Detailed Explanation
In summary, oxidation and reduction are two sides of the same chemical reaction. When we talk about redox reactions, we are merely discussing the transfer of oxygen or hydrogen between substances, defining their chemical changes.
Examples & Analogies
Just like a dance where partners switch roles, in redox reactions, one substance takes on the role of gaining while the other performs the role of losing, making it a perfect dance of chemistry.
Key Concepts
-
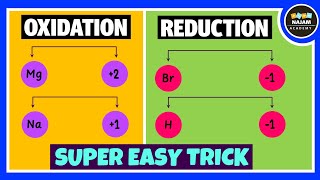
Oxidation: The process of gaining oxygen or losing electrons.
-
Reduction: The process of losing oxygen or gaining electrons.
-
Redox Reaction: A reaction involving both oxidation and reduction.
-
Corrosion: The deterioration of metals due to oxidation.
-
Rancidity: The oxidation of fats and oils resulting in rancid smells.
Examples & Applications
The oxidation of copper powder in the presence of heat forms copper(II) oxide.
Zinc oxide reduced by carbon to produce zinc and carbon monoxide.
In respiration, glucose is oxidized for energy.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Oxidation means a gain, Reduction it's a loss we gain.
Stories
Imagine a hero who seeks the light; it's oxidation when he gains oxygen bright. The villain in the tale loses his might; it's reduction in the dark, away from light.
Memory Tools
Remember 'OIL RIG': Oxidation Is Loss, Reduction Is Gain.
Acronyms
Use 'REDOX' to remember that Red is for Reduction and Ox is for Oxidation.
Flash Cards
Glossary
- Oxidation
The process of a substance gaining oxygen or losing hydrogen.
- Reduction
The process of a substance losing oxygen or gaining hydrogen.
- Redox Reaction
A chemical reaction where oxidation and reduction occur simultaneously.
- Corrosion
The wearing away of metals due to chemical reactions, often involving oxidation.
- Rancidity
The oxidation of fats and oils leading to unpleasant odors and tastes.
Reference links
Supplementary resources to enhance your learning experience.
