Classification
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll explore hydrocarbons, which are essential compounds consisting solely of hydrogen and carbon. Can anyone tell me why they are important in our daily lives?

They are used as fuels like LPG and CNG, right?

Exactly! Hydrocarbons are major sources of energy. They serve various industrial purposes as well. Now, we classify hydrocarbons. What do you think would be the first type?

Maybe saturated hydrocarbons?

Correct! Saturated hydrocarbons, like alkanes, only have single bonds. Let's remember that as 'Single is Stable' for saturated hydrocarbons.
Types of Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

There are three primary classifications: saturated, unsaturated, and aromatic. Can anyone give me examples of unsaturated hydrocarbons?

Alkenes and alkynes!

Right! Alkenes have double bonds, and alkynes have triple bonds. Remember 'Double is Kind (Alkene)' and 'Triple is Trouble (Alkyne)' to differentiate them.

Why are aromatic hydrocarbons unique?

Great question! Aromatic hydrocarbons, like benzene, are stable due to resonance. We'll explore resonance further in this chapter.
Physical and Chemical Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss the physical and chemical properties. How do you think alkanes behave compared to alkenes should they react with acids or bases?

Alkanes are usually more stable and don’t react much, right?

Exactly! Alkanes are quite inert. In contrast, alkenes and alkynes are more reactive due to their double and triple bonds. 'Saturated Stays Still, Unsaturated Unleashes!' Let's keep that in mind.

I see! So we have different reactivities depending on the type.

Precisely! Each class behaves differently, which we’ll further elucidate in subsequent lessons.
Identification of Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s focus on identifying hydrocarbons. Who can recall how to determine the structure of a hydrocarbon?

I think we look at the number of carbon-carbon bonds.

That's right! The bond type gives us an insight into whether it's an alkane, alkene, or alkyne. How about the cyclic structure? What do we call those?

Those are called cycloalkanes!

Great! Cycloalkanes are also saturated. Remember: 'Cycles Cycle Calmly'.

Can we build models to help visualize these structures?

Absolutely! Building models can reinforce these concepts.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Hydrocarbons are classified into saturated, unsaturated, and aromatic types according to the nature of their carbon-carbon bonds. This section covers the definitions of alkanes, alkenes, alkynes, and aromatic hydrocarbons, explaining their structures, properties, and importance in everyday applications.
Detailed
Detailed Summary of Classification of Hydrocarbons
Hydrocarbons are organic compounds consisting of only carbon and hydrogen. They are essential in various applications, including fuels and materials. This section classifies hydrocarbons into three categories:
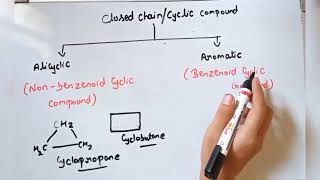
- Saturated Hydrocarbons: These contain only single bonds between carbon atoms. Alkanes (e.g., methane, ethane) are the primary examples. In the case of open-chain structures, they form a linear arrangement, while closed chains lead to cycloalkanes.
- Unsaturated Hydrocarbons: These hydrocarbons have at least one double (alkenes) or triple bond (alkynes) between carbon atoms, making them reactive. They can form various isomers, which differ in the arrangement of carbon atoms.
- Aromatic Hydrocarbons: These compounds contain ring structures with alternating double bonds, such as benzene. They exhibit unique stability due to resonance and can be mono- or disubstituted based on the substituents attached.
In This Section, You Will:
- Recognize the distinct types of hydrocarbon bonds.
- Write and identify the structure of isomers.
- Learn about the preparation methods for hydrocarbons.
- Differentiate physical and chemical properties of alkanes, alkenes, alkynes, and aromatic hydrocarbons.
- Understand the conformational variations among alkanes and the significance of resonance in aromatic compounds.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Types of Hydrocarbons
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Hydrocarbons are of different types. Depending upon the types of carbon-carbon bonds present, they can be classified into three main categories – (i) saturated, (ii) unsaturated, and (iii) aromatic hydrocarbons.
Detailed Explanation
Hydrocarbons are compounds composed only of carbon and hydrogen. They are classified based on the types of bonds between carbon atoms:
1. Saturated Hydrocarbons: These contain only single bonds (C-C and C-H), such as alkanes.
2. Unsaturated Hydrocarbons: These have double or triple bonds, such as alkenes (double bonds) and alkynes (triple bonds).
3. Aromatic Hydrocarbons: These are special cyclic compounds with a unique stability due to resonance, like benzene.
Examples & Analogies
Imagine hydrocarbons structured like a family tree:
- Saturated hydrocarbons are the solid branches: stable and strong because they only connect with single bonds (like a family tightly linked without any complex relationships).
- Unsaturated hydrocarbons are like branches that have bends (double bonds) or forks (triple bonds): flexible but less stable because the connections are not as strong as the solid branches.
- Aromatic hydrocarbons are the unique, twisted branches that are surprisingly stable due to their special structure; they have a charm of their own, captivating like a mysterious family story.
Saturated Hydrocarbons
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Saturated hydrocarbons contain carbon-carbon and carbon-hydrogen single bonds. If different carbon atoms are joined together to form open chains with single bonds, they are termed as alkanes. If carbon atoms form closed chains, they are called cycloalkanes.
Detailed Explanation
Saturated hydrocarbons only have single bonds, which makes them less reactive. Alkanes are the simplest form of saturated hydrocarbons with straight or branched chains (like propane or butane). Cycloalkanes are similar but arranged in ring structures (like cyclohexane). Each carbon atom in these compounds is bonded to the maximum number of hydrogen atoms possible.
Examples & Analogies
Think of saturated hydrocarbons like a fully packed concert hall, where everyone has a seat (a hydrogen atom) without any empty spots (bonds). The more people you fit in without leaving gaps (single bonds), the less room there is for any additional guests (reactivity). Each person at the concert is happy and stable sitting tightly together.
Unsaturated Hydrocarbons
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Unsaturated hydrocarbons contain carbon-carbon multiple bonds – double bonds, triple bonds, or both. They are more reactive compared to saturated hydrocarbons.
Detailed Explanation
Unsaturated hydrocarbons have either double or triple bonds between carbon atoms, which means fewer hydrogen atoms are attached to the carbon chain. This creates 'open spaces' that make them more reactive as they can participate in reactions to form new compounds. Examples include alkenes (with double bonds) and alkynes (with triple bonds).
Examples & Analogies
Imagine a restaurant full of group discussions; each double bond represents a discussion that interrupts the flow. The more discussions (double or triple bonds) you have, the more interactions and excitement happen compared to a calm table with everyone seated and happy (saturated hydrocarbons). Hence, the lively discussions mean more potential for reactions in cooking styles!
Aromatic Hydrocarbons
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Aromatic hydrocarbons are a special type of cyclic compound. They have a planar ring structure and are stabilized by resonance.
Detailed Explanation
Aromatic hydrocarbons are characterized by their cyclic structure, often with alternating double bonds, like benzene. However, unlike simple alkenes, they display resonance, meaning the electrons are delocalized across the ring. This makes them remarkably stable and less reactive than their unsaturated counterparts.
Examples & Analogies
Imagine a circular dance floor where everyone is constantly shifting their partner but always staying on the floor. Each dancer (electron) creates beautiful patterns without ever stepping off the floor (circular structure). This 'dance of electrons' is what makes aromatic compounds incredibly stable and unique in reactions.
Modeling Hydrocarbons
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
You can construct a large number of models of hydrocarbons, both open-chain and closed-chain, keeping in mind that carbon is tetravalent and hydrogen is monovalent.
Detailed Explanation
Modeling hydrocarbons can help visualize their structures. Carbon atoms can form four bonds due to their tetravalency (four electrons in the outer shell), while hydrogen can only form one bond. Therefore, when constructing models, use materials like toothpicks for bonds and balls for atoms to represent these principles practically.
Examples & Analogies
Think of building with blocks: carbon atoms are like sturdy building blocks that can connect in many ways (tetravalent), while hydrogen atoms are smaller blocks that fit onto the carbon blocks. Using different colors can help differentiate between types of atoms, making learning about their bonds and structures interactive and engaging.
Key Concepts
-
Hydrocarbons: Compounds solely made of carbon and hydrogen.
-
Saturated Hydrocarbons: Only contain carbon-carbon single bonds.
-
Unsaturated Hydrocarbons: Contain carbon-carbon double or triple bonds.
-
Aromatic Hydrocarbons: Compounds with stable resonance structures.
Examples & Applications
Methane (CH4) as an example of a saturated hydrocarbon.
Ethylene (C2H4), a common unsaturated hydrocarbon.
Benzene (C6H6), illustrating aromatic hydrocarbon properties.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Feel free to play with bonds today, single for alkanes, triple for an alkyne’s way.
Stories
Imagine a city of hydrocarbons: Alkanes live in peaceful, single-story homes, Alkenes enjoy the spirited double dancing, while Alkynes throw lively triple bond parties.
Memory Tools
Remember: S for Saturated, U for Unsaturated, and A for Aromatic in hydrocarbons.
Acronyms
Use the acronym S.U.A. to remember Saturated, Unsaturated, and Aromatic hydrocarbons.
Flash Cards
Glossary
- Hydrocarbon
An organic compound consisting exclusively of carbon and hydrogen.
- Saturated Hydrocarbon
Hydrocarbons composed solely of single bonds.
- Unsaturated Hydrocarbon
Hydrocarbons containing at least one double or triple bond.
- Aromatic Hydrocarbon
Cyclic hydrocarbons characterized by resonance stability, commonly containing a benzene ring.
Reference links
Supplementary resources to enhance your learning experience.
