Preparation of Benzene
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Cyclic Polymerization of Ethyne
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore how ethyne, a simple alkyne, can be converted into benzene through cyclic polymerization. This process allows multiple ethyne molecules to join together to form the aromatic structure of benzene. Can anyone tell me the importance of benzene in organic chemistry?

Benzene is used as a starting material for many chemical syntheses, like dyes and pharmaceuticals.

Exactly! Benzene’s structure and stability make it a valuable intermediate in many reactions. The cyclic polymerization of ethyne highlights the transition from unsaturated to aromatic compounds.

Does ethyne need any special conditions to undergo this polymerization?

Good question! Yes, typically, high temperature and pressure are needed to facilitate this reaction. Additionally, catalysts can increase the efficiency of the polymerization. Remember, ethyne is also a key component in producing various hydrocarbons.

So, can we say that ethyne undergoes a significant transformation to become benzene?

Absolutely! It’s a prime example of how simple molecules can form complex structures.

Now, let’s summarize what we learned: Ethyne can convert to benzene through cyclic polymerization, a crucial reaction highlighting the significance of structural transformation in organic chemistry.
Decarboxylation of Aromatic Acids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we’ll discuss the decarboxylation of aromatic acids. Can anyone explain what decarboxylation entails?

It involves the removal of a carboxyl group from a compound.

Exactly! In this case, sodium salts of benzoic acid are heated with soda lime. What do you think the product will be when these react?

Benzene should be formed, right?

Correct! This reaction is a great example of transforming carboxylic acids into hydrocarbons, showcasing the versatility of organic reactions. Can anyone think of why this might be an advantageous reaction?

It provides a way to create carbon-rich compounds from raw materials.

Exactly! You all are grasping the concepts well. To sum up: Decarboxylation of sodium benzoate results in the formation of benzene, illustrating the utility of carboxylic acids in organic synthesis.
Reduction of Phenol
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s look at how we can reduce phenol to obtain benzene. What do you think the reduction process involves?

It might involve removing an oxygen atom.

That’s partially correct! Phenol can be reduced by passing its vapors over heated zinc dust. This process removes the hydroxyl group. What is the implication of this reduction?

It shows that we can convert functional groups to simpler hydrocarbons.

Exactly! Understanding such reductions is crucial in organic synthesis. Can anyone provide an example of where this knowledge of reduction might be applied?

We could use it to create hydrocarbons for fuels or chemical intermediates.

That’s a clever connection! To conclude, reducing phenol with zinc dust effectively transforms it into benzene, underscoring the methods we have for synthesizing aromatic compounds.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
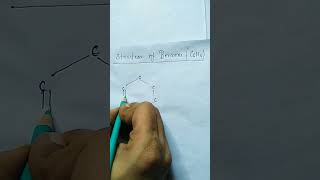
Benzene, an important aromatic hydrocarbon, can be prepared through several methods in the laboratory. Notably, it can be synthesized via cyclic polymerization of ethyne, decarboxylation reactions involving sodium salts of aromatic acids, and by reducing phenol with zinc dust. These methods highlight the versatility of benzene synthesis and its significance in organic chemistry.
Detailed
Preparation of Benzene
Benzene (C6H6) is a significant aromatic hydrocarbon widely used in chemical synthesis and industry. It can be isolated commercially from coal tar, but laboratory synthesis methods expand its availability and application. This section explores three major methods for preparing benzene:
1. Cyclic Polymerization of Ethyne
Ethyne, also known as acetylene, can undergo a process called cyclic polymerization to form benzene. This method highlights the structural transformation of simple hydrocarbons into more complex aromatic compounds.
2. Decarboxylation of Aromatic Acids
Another method involves the decarboxylation of sodium salts of benzoic acid. When heated with soda lime (a mixture of sodium hydroxide and calcium oxide), benzene is formed as carbon dioxide is eliminated from the carboxylic acid group.
This method demonstrates the conversion of carboxylic acids to hydrocarbons, further illustrating the versatility of organic synthesis.
3. Reduction of Phenol
Finally, phenol can be reduced to benzene by passing its vapors over heated zinc dust. This reduction process illustrates how functional groups can be removed to yield simpler aromatic structures.
These methods of preparing benzene are vital for various chemical applications, ensuring its importance in organic chemistry.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Cyclic Polymerization of Ethyne
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
(i) Cyclic polymerisation of ethyne: (Section 9.4.4)
Detailed Explanation
The synthesis of benzene can occur through the cyclic polymerization of ethyne. This process involves the formation of benzene from ethyne molecules by creating a cyclic structure. Ethyne, known chemically as acetylene, is a simple alkyne which can polymerize under certain conditions. When multiple ethyne molecules are subjected to the right temperature and pressure, they can bond together to form a cyclic compound, resulting in the production of benzene.
Examples & Analogies
Think of making a necklace from individual beads. When you take several beads (ethyne) and thread them into a loop, you create a beautiful necklace (benzene). Just as adding more beads creates a longer chain, adding more ethyne molecules can create a larger ring structure, ultimately resulting in benzene.
Decarboxylation of Aromatic Acids
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
(ii) Decarboxylation of aromatic acids: Sodium salt of benzoic acid on heating with sodalime gives benzene.
Detailed Explanation
Another method to prepare benzene is through decarboxylation of sodium benzoate. This process involves a chemical reaction that eliminates a carboxyl group (COOH) from the aromatic acid, leading to the formation of benzene. When sodium benzoate is heated with sodalime (a mixture of sodium hydroxide and calcium oxide), the carboxyl group is removed, and benzene is produced as a result.
Examples & Analogies
Imagine you have a balloon (sodium benzoate) filled with a gas (the carboxyl group). When you heat the balloon, it expands and eventually pops, releasing the gas and leaving you with just the balloon (benzene) remaining. The heating causes the gas (the carboxyl group) to escape, thus forming benzene from the aromatic acid.
Reduction of Phenol
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
(iii) Reduction of phenol: Phenol is reduced to benzene by passing its vapours over heated zinc dust.
Detailed Explanation
The reduction of phenol to yield benzene represents another preparatory method. In this process, phenol is subjected to reduction, which involves the removal of an oxygen atom. By passing the vapors of phenol over heated zinc dust, the phenol loses its hydroxyl group (OH), resulting in the formation of benzene. This method is efficient and direct, transforming a functionalized aromatic compound into a simpler aromatic compound.
Examples & Analogies
You can think of this process like peeling a fruit (phenol) to enjoy its fresh and pure inside (benzene). Just as you remove the peel to get to the juicy fruit, you remove the hydroxyl group from phenol to produce benzene, creating a purer form of the aromatic compound.
Key Concepts
-
Cyclic Polymerization: A method converting ethyne to benzene by joining multiple ethyne molecules in a cyclic manner.
-
Decarboxylation: A reaction converting sodium benzoate to benzene by eliminating carbon dioxide.
-
Reduction of Phenol: The transformation of phenol to benzene by removal of -OH group through reduction.
Examples & Applications
Cyclic Polymerization: Ethyne (C2H2) forms benzene through cyclic polymerization under high temperature.
Decarboxylation: Sodium benzoate with soda lime yields benzene by eliminating carbon dioxide.
Reduction: Passing phenol (C6H5OH) over heated zinc dust produces benzene.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ethyne polymerizes in a cycle, forming benzene's delightful title.
Stories
Imagine ethyne gathered for a party. When heated, they hold each other tight, forming a benzene ring with delight.
Memory Tools
Benzene: B for Benzene, C for Cycle, D for Decarboxylation.
Acronyms
B.E.R. - Benzene from Ethyne, Reduction of Phenol, Decarboxylation.
Flash Cards
Glossary
- Benzene
An aromatic hydrocarbon with the molecular formula C6H6, formed from cyclic structures.
- Cyclic Polymerization
A reaction where unsaturated hydrocarbons join to form cyclic structures, like benzene from ethyne.
- Decarboxylation
The removal of a carboxyl group from a compound, resulting in the release of carbon dioxide.
- Soda Lime
A mixture of sodium hydroxide and calcium oxide used in organic chemistry for reactions like decarboxylation.
- Reduction
A chemical reaction that involves the gain of electrons or decrease in oxidation state, often resulting in the removal of oxygen or adding hydrogen.
Reference links
Supplementary resources to enhance your learning experience.
