Exercises
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Nomenclature of Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to learn about how to properly name hydrocarbons. Remember, there are specific rules we follow for each type of hydrocarbon!

What are the main types of hydrocarbons we need to know?

Great question! We primarily work with alkanes, alkenes, alkynes, and aromatic hydrocarbons. Each has its own set of rules.

Can you give us an example of naming an alkane?

Of course! For example, the alkane with three carbon atoms is called propane. The general formula for alkanes is CnH2n+2.

What about alkenes? How do they differ?

Alkenes have at least one double bond, so their formula is CnH2n. For instance, C2H4 is known as ethene.

How do we identify the longest carbon chain again?

You want to find the maximum number of carbon atoms connected in a continuous line. This chain gives you the base name of the alkane. Then we number it so that substituents have the lowest possible numbers.

Today, we introduced some key nomenclature principles for hydrocarbons.
Understanding Isomerism
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand naming, let\u2019s dive into isomers, which are compounds with the same formula but different structures or configurations.

What types of isomers are we looking at?

That's right! We primarily focus on structural isomers and geometrical isomers.

How can we create structural isomers for C4H10?

For that formula, we have two main isomers: butane, which is a straight-chain, and isobutane, which is branched. Let\u2019s draw them out!

Is circular chain formation possible too?

Excellent point! Cyclic forms are also possible, as seen with cycloalkanes such as cyclobutane. That\u2019s another form of isomerism.

Today, we understood structural isomers, identified examples, and practiced drawing them.
Physical Properties of Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let\u2019s explore the physical properties of hydrocarbons. How do structure and molecular size impact these properties?

Do smaller hydrocarbons behave differently than larger ones?

Absolutely! Smaller alkanes like methane and ethane are gases, while larger ones are liquids and solids.

What about solubility?

Hydrocarbons are generally non-polar and insoluble in water. They dissolve well in non-polar solvents. Always remember: 'like dissolves like'.

Can we see the same trends in boiling points?

Exactly! As molecular size increases, boiling points typically increase due to increased van der Waals forces between molecules.

Today, we uncovered the relationship between molecular structure and physical properties.
Chemical Properties of Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's look at the chemical properties of hydrocarbons. What types of reactions do they commonly undergo?

I remember something about combustion reactions!

Yes, exactly! Alkanes, alkenes, and alkynes can all undergo combustion, producing carbon dioxide and water.

What are some other reactions?

Hydrocarbons can also undergo substitution, addition, and polymerization reactions. For example, alkenes readily engage in addition reactions due to their double bonds.

Why are they used as fuels?

Hydrocarbons release a huge amount of energy during combustion, thus making them excellent energy sources.

Today, we focused on how hydrocarbons react with various reagents and how they can be utilized as fuels.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Exercises in this section help students solidify their understanding of hydrocarbons by addressing nomenclature, structural isomerism, properties, reactions, and applications in real-world scenarios. Each exercise aims to test student knowledge through questions of varying complexity, ensuring a comprehensive grasp of the subject matter.
Detailed
Detailed Summary his section presents a series of exercises designed to reinforce the key concepts covered in the chapter on hydrocarbons. Students will engage with the nomenclature rules defined by IUPAC for alkanes, alkenes, alkynes, and aromatic hydrocarbons. Emphasis is placed on understanding structural isomerism through practical exercises where students will draw different isomers for given formulae. The section also challenges students to differentiate the physical and chemical properties of the various hydrocarbons and to apply these concepts in predicting reaction outcomes. Additionally, questions addressing the industrial and everyday importance of hydrocarbons provide context to their relevance, encouraging students to understand hydrocarbons not just theoretically but also in practical applications.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Formation of Ethane during Chlorination of Methane
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
How do you account for the formation of ethane during chlorination of methane?
Detailed Explanation
In the chlorination of methane, chlorine reacts with methane (CH₄) to replace one of the hydrogen atoms with a chlorine atom, forming chloromethane (CH₃Cl). Additionally, if methyl radicals are formed in the reaction, they can react with another molecule of methane, leading to the formation of ethane (C₂H₆). This reaction can be understood through the free radical mechanism, where the initial chlorine radical abstracts a hydrogen atom from methane, followed by possible recombination of the radicals.
Examples & Analogies
Think of it like a group of friends (hydrogens) playing a game where one friend (chlorine) wants to join. As one friend gets picked to play with the new friend, the original group loses a member but can invite a nearby friend to form a new duo (ethane).
Naming Organic Compounds
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
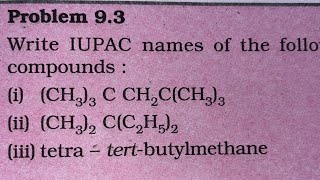
Write IUPAC names of the following compounds: (a) CH₃CH=C(CH₃)₂ (b) CH₂=CH-C≡C-CH₃ (c) (d) –CH₂–CH₂–CH=CH₂ (e) (f) CH₃(CH₂)₄ CH(CH₂)₃ CH₃ CH₂–CH(CH₃)₂ (g) CH₃ – CH = CH – CH₂ – CH = CH – CH₂ – CH = CH₂ | C₂H₅
Detailed Explanation
In IUPAC naming, the longest carbon chain containing the maximum functional groups is identified. Each compound given is broken down as follows:
(a) CH₃CH=C(CH₃)₂: This compound has a double bond and a branched chain. The name is 2-methylbut-2-ene.
(b) CH₂=CH-C≡C-CH₃: This compound has both double and triple bonds. It is named buta-1,3-diene.
(c) and (d) will follow similar logic to identify the functional groups and correct naming conventions.
Examples & Analogies
Naming organic compounds is much like labeling shelves in a store. You want to ensure everything is easy to find based on the main item, and additional items are labeled neatly for clarity, just like how functional groups determine the name of a compound.
IUPAC Names and Isomer Structures
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bonds as indicated: (a) C₄H₈ (one double bond) (b) C₅H₈ (one triple bond)
Detailed Explanation
When dealing with isomers, you have to consider various structural arrangements. For C₄H₈ with one double bond, it can exist as but-1-ene, but-2-ene, or as a cyclic compound like cyclobutene. For C₅H₈ with one triple bond, possible structures include pent-1-yne, pent-2-yne, or isomers like 3-methylbut-1-yne.
Examples & Analogies
Creating isomers is akin to rearranging building blocks. You can configure the blocks in various ways, leading to distinct structures, much like how different arrangements of carbon atoms lead to unique properties in organic compounds.
Ozonolysis of Alkenes
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Write IUPAC names of the products obtained by the ozonolysis of the following compounds: (i) Pent-2-ene (ii) 3,4-Dimethylhept-3-ene (iii) 2-Ethylbut-1-ene (iv) 1-Phenylbut-1-ene
Detailed Explanation
Ozonolysis is a reaction where ozonolysis products are formed through cleavage of double bonds in alkenes. In pent-2-ene, ozonolysis yields two molecules of aldehydes. The remaining compounds similarly break down under ozonolysis, producing various aldehydes or ketones, depending on the structure of the original alkene.
Examples & Analogies
Consider ozonolysis like cutting a sandwich filled with assorted spreads and fillings. Depending on where you cut (the type of alkene), you get different halves of the sandwich (products), which are unique but derived from the same whole.
Combustion Reactions of Hydrocarbons
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Write chemical equations for combustion reaction of the following hydrocarbons: (i) Butane (ii) Pentene (iii) Hexyne (iv) Toluene
Detailed Explanation
Combustion reactions involve hydrocarbons reacting with oxygen to produce carbon dioxide and water, releasing heat. For example, combustion of butane (C₄H₁₀) can be represented as: C₄H₁₀ + 5O₂ → 4CO₂ + 5H₂O. Similarly, pentene and hexynes would follow the same basic pattern but with different coefficients based on their carbon counts.
Examples & Analogies
Think of hydrocarbon combustion as a campfire. As you add logs (hydrocarbons) and oxygen (air), they ignite, creating flames and warmth (CO₂ and H₂O). Each type of log burns differently, just like different hydrocarbons release energy differently.
Key Concepts
-
Importance of hydrocarbons as energy sources and raw materials in the industry.
-
Classification of hydrocarbons into alkanes, alkenes, alkynes, and aromatics.
-
Nomenclature rules for naming hydrocarbons.
-
Understanding isomerism, particularly structural isomerism.
Examples & Applications
Ethane (C2H6) being an alkane
Ethene (C2H4) as an alkene
Ethyne (C2H2) as an alkyne
Benzene (C6H6) as an aromatic compound
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hydrocarbons long and short, From fuels to plastics, they warp and distort.
Stories
Imagine hydrocarbons as energetic characters in a race to combine and form larger families, each vying for energy production.
Memory Tools
Acronym 'NASH' to remember - Nomenclature, Alkanes, Structuring, Hydrocarbons.
Acronyms
Use 'PHA' to remember four basic classification of hydrocarbons
for Paraffins (Alkanes)
for Alkenes
for Alkynes.
Flash Cards
Glossary
- Hydrocarbon
Compounds containing only hydrogen and carbon.
- Alkane
Saturated hydrocarbons with only single bonds.
- Alkene
Unsaturated hydrocarbons with at least one double bond.
- Alkyne
Unsaturated hydrocarbons with at least one triple bond.
- Aromatic Hydrocarbon
Compounds that contain a benzene ring.
- Isomer
Compounds with the same molecular formula but different arrangements of atoms.
- Nomenclature
The system of naming chemical compounds.
- Substitution Reaction
A reaction where one atom or group of atoms is replaced by another.
- Addition Reaction
A reaction where atoms are added to a molecule without the loss of any atom.
Reference links
Supplementary resources to enhance your learning experience.
