Abnormal Molar Mass
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Molar Mass
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing molar mass. Can anyone tell me what molar mass means?

Is it the mass of one mole of a substance?

Exactly! The molar mass is the mass of one mole of a substance, typically expressed in grams per mole. This measurement is crucial to understanding concentrations in solutions. Now, does anyone know how molar mass can vary in solutions?

It might change if the substance dissolves and dissociates, right?

Great observation! Let's explore how this happens, particularly focusing on abnormal molar mass.

Remember, the formula for molar mass can be affected by ionic dissociation and molecular association.

What's the difference between dissociation and association?

Dissociation refers to ionic compounds breaking into ions, increasing particle count in the solution. Association is when molecules join to form larger particles, decreasing the count. Both scenarios influence molar mass.

So if normal molar mass is 100 g/mol, and the van’t Hoff factor is greater than 1, we would see a lower observed molar mass?

Exactly! That’s a perfect application of the van’t Hoff factor (i), which helps us quantify these effects. To tie this together, the abnormal molar mass is defined in terms of how many particles are present in solution compared to before.

Can anyone summarize the key takeaways from today’s session?

The molar mass can change based on how solutes behave in solutions, either increasing or decreasing particle count.

Exactly correct! Great job, everyone!
Colligative Properties and Their Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We’ve covered abnormal molar mass. Now let's discuss colligative properties. Who remembers what colligative properties are?

They're properties that depend on the number of solute particles in a solution?

Exactly right! Examples include boiling point elevation and freezing point depression. How do you think these are affected by the van’t Hoff factor?

If more particles are present due to dissociation, the change in boiling or freezing points would be larger!

Yes! More particles increase the effect on boiling and freezing points. For example, when we calculate the elevation in boiling point, it's modified as \( \Delta T_b = iK_bm \). Can someone expand on what this means?

We need the molality and the constant for the solvent to find the change in boiling point.

That's right! We combine these to find how temperature impacts our solutions. Let's remember this formula: it helps us understand how solutes behave in solutions.

Could we also use this in real-life examples, like in cooking?

Absolutely! Understanding high concentrations can prevent freezing in ice cream or enhance flavor in soups. Can anyone summarize today’s discussion?

Colligative properties depend on particle number, and the van’t Hoff factor is crucial in determining how much change occurs.

Great summary! I'm glad to see everyone engaged!
Applications in Various Fields
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s talk about how abnormal molar mass impacts various industries. Can anyone think of an example?

Well, in pharmaceuticals, understanding molar mass helps in creating effective drug dosages.

Great example! It's essential in ensuring that solutions maintain appropriate concentrations for health and safety. How about in food science?

We use the understanding of boiling points to preserve food!

Exactly! And remember how freezing point depression is applied in making ice cream? How does that relate to our discussions?

If we can manage the molar mass, we can control the texture and consistency of the ice cream!

Yes! It’s fascinating how chemistry relates to everyday life. Can anyone sum up today’s insights?

Abnormal molar mass has crucial applications in medicine and food science through its impact on colligative properties.

Excellent summary! Understanding these principles can open doors to many scientific advancements!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides a comprehensive overview of how the abnormal molar mass differs from the normal molar mass based on the degree of molecular interaction (dissociation or association) in solutions. It introduces the van’t Hoff factor (i) and explores how colligative properties and the calculated molarity help in understanding such effects.
Detailed
Detailed Summary of Abnormal Molar Mass
In this section, we explore the concept of abnormal molar mass in solutions, emphasizing two key phenomena: ionic dissociation and molecular association. When a solute dissolves in a solvent, the expected molar mass may differ from the observed due to these interactions.
- Ionic Dissociation: When ionic compounds like KCl dissolve in water, they dissociate into their constituent ions (K+ and Cl-). This leads to an increase in the number of particles in the solution, resulting in a lower observed molar mass than the true molar mass.
- Molecular Association: Conversely, certain solutes may associate in solutions, such as ethanoic acid in benzene, leading to fewer particles than expected. This results in an observed molar mass that appears higher than the actual molar mass.
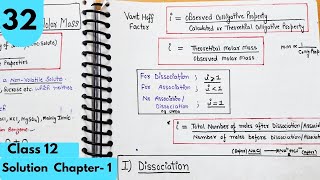
To quantify these effects, van’t Hoff introduced the van’t Hoff factor (i), defined as the ratio between the normal molar mass and the abnormal molar mass. The factor aids in modifying equations for colligative properties like depression of freezing point and elevation of boiling point:
- For relative lowering of vapor pressure:
\[ \frac{p_0 - p}{p_0} = i \cdot \frac{n_2}{n_1} \]
- For boiling point elevation and freezing point depression:
\[ \Delta T_b = i imes K_b imes m \]
- For osmotic pressure:
\[ P = i \cdot \frac{n_2}{V} \cdot R imes T \]
This section elucidates the importance of differentiating between normal and abnormal molar mass in practical applications such as determining concentrations in various solutions, which has further implications in chemical processes and biological systems.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Abnormal Molar Mass
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A solution is a homogeneous mixture of two or more substances. Solutions are classified as solid, liquid, and gaseous solutions. The concentration of a solution is expressed in terms of mole fraction, molarity, molality, and percentages. The dissolution of a gas in a liquid is governed by Henry’s law, according to which, at a given temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas.
Detailed Explanation
This chunk introduces the concept of a solution and how it can be formed from different types of substances—solids, liquids, and gases. Importantly, it outlines that solutions have defined concentrations that can be expressed in several ways, including mole fraction (the ratio of the amount of solute to the total amount of solution), molarity (the number of moles of solute per liter of solution), and molality (the number of moles of solute per kilogram of solvent). These definitions help us understand how concentrations are used to quantify solutions in chemistry.
Examples & Analogies
Think of a solution like a fruit smoothie. The fruits (or solutes) are blended into the juice (or solvent) to create a uniform drink (solution). Just as we can have different combinations of fruits to make various smoothies, we can combine different solutes to make a variety of solutions, and we can describe the strength of these smoothies in different ways (e.g., one fruit smoothie may have a high concentration of strawberries while another has more bananas).
Henry's Law
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Henry’s law states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of the liquid or solution.
Detailed Explanation
Henry's Law is vital for understanding how gases dissolve in liquids. The law implies that if you increase the pressure of a gas above a liquid, more gas molecules will enter the liquid until the system reaches a new equilibrium. This is particularly evident in carbonated drinks; they are bottled under high pressure to keep the carbon dioxide gas dissolved in the liquid. When you open the bottle, the pressure is released, and gas bubbles escape, which is why you see fizz.
Examples & Analogies
Imagine a sealed bottle of soda. When it's sealed, the pressure inside keeps a lot of carbon dioxide gas mixed into the soda. When you open it, the pressure drops, and suddenly, the gas forms bubbles and escapes into the air. This is Henry's law in action—solubility changes with pressure.
Raoult's Law and Colligative Properties
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Raoult’s law states that the partial vapor pressure of each component of a solution is directly proportional to its mole fraction present in the solution. According to Raoult’s law, when a non-volatile solid solute is added to a solvent, the vapor pressure of the solvent decreases.
Detailed Explanation
Raoult's Law helps describe the behavior of solutions when a non-volatile solute is added. For example, when sugar is dissolved in water, the water molecules at the surface can escape into the vapor phase less easily because some of the surface is now occupied by sugar molecules. This results in a lower vapor pressure compared to pure water. These changes in vapor pressure lead to colligative properties, which include boiling point elevation and freezing point depression—properties that depend on the number of solute particles rather than their nature.
Examples & Analogies
Think about making a syrup for pancakes. When you add sugar to water to make the syrup, it not only sweetens the water but also changes its boiling and freezing points. Adding sugar raises the boiling point of the water, meaning it takes longer to boil, just like when you add salt to water, it boils at a higher temperature. This is why syrup can remain liquid even when it's colder than regular water.
Van't Hoff Factor
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The van’t Hoff factor, symbolized as i, helps quantify the degree of dissociation or association of solutes in solution, allowing for calculations related to colligative properties.
Detailed Explanation
The van't Hoff factor is an important concept in understanding how solutes behave in solution. It is particularly useful for strong electrolytes that dissociate into ions, like sodium chloride (NaCl), which breaks into Na⁺ and Cl⁻ in solution, effectively doubling the number of 'particles' in the solution. The factor i helps refine calculations by accounting for the changes in colligative properties caused by the solute's behavior—either the expected molar mass is lowered for dissociating solutes or raised for associating ones.
Examples & Analogies
Consider how some companies sell concentrated drinks as powders to be mixed with water. When you add a powder to water, if it dissolves into multiple components (like in the case of salt), it can make the drink taste stronger than expected just because there are more 'taste molecules' in the solution. The van't Hoff factor helps us understand and predict how much flavor you’ll taste based on what happens when that powder is added into your drink!
Key Concepts
-
Abnormal Molar Mass: Refers to the difference in expected vs. observed molar mass due to solute behavior.
-
Van't Hoff Factor (i): Quantifies the extent of dissociation or association in a solution.
-
Colligative Properties: Properties that depend solely on the number of solute particles in a solution.
Examples & Applications
The dissociation of sodium chloride in water leading to increased particle count and lower observed molar mass.
The association of ethanoic acid molecules in benzene resulting in fewer effective solute particles than expected, raising the molar mass.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Molar mass can shift, with solutes in a rift, Dissociating, associating, gives us a gift.
Stories
Once upon a time, in a lab, a chemist discovered that when ionic bonds break, they become more numerous, leading to lower mass, while some molecules would join hands, raising their collective weight—a tale of discovery in solutions!
Memory Tools
Remember 'i' for van't Hoff: Increased particles from ions, or Indexed the decrease with dimer bonds.
Acronyms
DISS (Dissociation, Increase, Solutions, Significance) helps to recall why molar masses change based on particle behavior.
Flash Cards
Glossary
- Abnormal Molar Mass
The observed molar mass which differs from the expected value due to dissociation or association of solute particles.
- Van't Hoff Factor (i)
A measure of the effect of dissociation or association of solute particles compared to the number of particles initially present.
- Colligative Properties
Properties of solutions that depend on the number of solute particles, not their identity.
- Dissociation
The process by which an ionic compound breaks apart into its individual ions in solution.
- Association
The process by which molecules aggregate to form larger particles in a solution.
Reference links
Supplementary resources to enhance your learning experience.
