Mass Percentage (w/w)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Percentage
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to discuss mass percentage, often noted as (w/w). Can anyone tell me what you understand by this term?

I think it's a way to express how much of a substance is in a solution.

Exactly! Mass percentage indicates the concentration of a solute in a solution. The formula for mass percentage is: \(\text{Mass %} = \frac{\text{Mass of solute}}{\text{Total mass of solution}} \times 100\). Can anyone give me a practical example of where this might be used?

I've seen it used in cleaning products indicating how much active ingredient there is.

Great example! Remember, applications like these are crucial in industries for safety and regulations.

To help you remember the formula, think of 'mass first, then total' — it follows a straightforward composition-based approach.
Calculating Mass Percentage
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's calculate the mass percentage together. Suppose we have 10 g of glucose mixed in 90 g of water. What would the total mass of the solution be?

That would be 100 g!

Correct! Now, using our formula, can anyone calculate the mass percentage of glucose in this solution?

It's \(\frac{10 g}{100 g} \times 100 = 10%\)!

Fantastic! It’s important to practice such calculations, as they lay the foundation for understanding other concentration units in chemistry.
Applications of Mass Percentage
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know how to calculate mass percentage, let’s explore its applications. Why do you think knowing the mass percentage is essential in pharmaceuticals?

It’s probably to ensure patients get the right dose of medication.

Exactly! Accurate dosage can drastically affect treatment outcomes. In what other industries is mass percentage critical?

In food and beverage, they need to indicate how much of a preservative is included.

Absolutely! Mass percentages ensure safety standards are met. As a mnemonic, remember 'mass matters.' It will help reinforce the importance of mass percentages!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
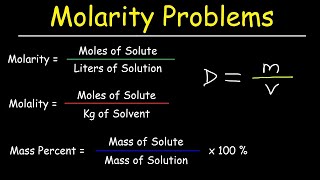
Mass percentage is a critical measurement in the chemistry of solutions, indicating the concentration of a solute in a solvent. The section covers its definition, formula for calculation, examples of its use in various industries, and the importance of accurate measurement.
Detailed
Mass Percentage (w/w)
Mass percentage, often denoted as (w/w), refers to the mass of a component in a solution relative to the total mass of the solution expressed as a percentage. It plays a vital role as a method of expressing solution concentration, especially in industrial applications where precision is paramount.
Formula
The formula for calculating mass percentage is:
$$\text{Mass % of a component} = \frac{\text{Mass of the component in the solution}}{\text{Total mass of the solution}} \times 100$$
Example Calculation
For instance, in a solution with 10 g of glucose dissolved in 90 g of water:
- Total mass of the solution = 10 g + 90 g = 100 g
- Mass % of glucose = (10 g / 100 g) × 100 = 10%
Importance
Mass percentage is particularly useful in contexts like pharmaceuticals, where the exact concentration of active ingredients is crucial for effectiveness and safety. It allows for easy comparisons between different solutions and is a standard measurement in chemical industries.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Mass Percentage
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The mass percentage of a component of a solution is defined as:
Mass % of a component
Mass of the component in the solution
Total mass of the solution
For example, if a solution is described by 10% glucose in water by mass, it means that 10g of glucose is dissolved in 90 g of water resulting in a 100 g solution.
Detailed Explanation
Mass percentage is a way to express how much of a specific component is present in a solution compared to the total mass of the solution. To calculate the mass percentage, you divide the mass of the solute (the substance being dissolved) by the total mass of the solution and multiply by 100 to get a percentage. In our example, if you have 10 grams of glucose dissolved in 90 grams of water, the total mass of the solution is 100 grams. Therefore, the mass percentage of glucose is (10g / 100g) × 100 = 10%.
Examples & Analogies
Think about a fruit drink you make at home. If you add 10 grams of sugar to 90 grams of water, you can think of it just like adding a scoop of sugar for sweetness. The drink (solution) contains 10% sugar, which means that if you take 100 grams of that fruit drink, 10 grams is sugar and 90 grams is water.
Application of Mass Percentage
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Concentration described by mass percentage is commonly used in industrial chemical applications. For example, commercial bleaching solution contains 3.62 mass percentage of sodium hypochlorite in water.
Detailed Explanation
Mass percentage is particularly useful in various industries where precise concentrations of substances are crucial for safety and effectiveness. In the example of a bleaching solution containing 3.62% sodium hypochlorite, it indicates that in every 100 grams of the solution, there are 3.62 grams of sodium hypochlorite. This measurement is essential for determining how strong the solution is and how much should be used for specific cleaning tasks.
Examples & Analogies
When you buy a bottle of bleach at the store, the label might tell you it contains a certain percentage of sodium hypochlorite. Knowing this percentage can help you understand how effective it will be at cleaning when you dilute it with water, similar to how knowing the percentage of alcohol in a bottle helps you understand how strong your drink is.
Conclusion on Mass Percentage
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Each method of expressing concentration of the solutions has its own merits and demerits. Mass %, ppm, mole fraction and molality are independent of temperature, whereas molarity is a function of temperature. This is because volume depends on temperature and the mass does not.
Detailed Explanation
Understanding that mass percentage has its own advantages, such as being temperature-independent, is crucial. This means that regardless of temperature changes, the mass percentage remains the same, making it a reliable measurement in many applications. In contrast, molarity (the concentration based on volume) will change with temperature as the volume of a liquid can expand or contract with temperature changes.
Examples & Analogies
Consider two identical containers filled with liquid; one with a high temperature and the other at room temperature. If you pour in sugar, the concentration in terms of mass percentage stays the same regardless of the temperature, but if you were to measure how much sugar you can dissolve based on volume (molarity), you'd find that as the temperature increases, you might get less sugar dissolved per liter in the hotter liquid. This makes mass percentage a more stable measure in many scenarios.
Key Concepts
-
Mass Percentage: A method of expressing the concentration of a solute in a solution.
-
Formula: Mass % = (mass of solute / total mass of solution) × 100
-
Importance in Industries: Used in pharmaceuticals, food, and chemical industries.
Examples & Applications
If 20 g of salt is dissolved in 80 g of water, the mass percentage of salt in the solution is (20 g / 100 g) × 100 = 20%.
A commercial bleaching solution containing 3.62 mass percentage of sodium hypochlorite in water.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Count your grams, watch the scales, mass percentage never fails!
Stories
Imagine a baker, mixing 10 cups of flour with 1 cup of sugar. The sugar’s story is told by its mass getting counted in the total mix for the best cake!
Memory Tools
Mass Percent = Part / Whole × 100, think ‘Part for Percent’ when calculating.
Acronyms
Remember M.P. Standard
Mass Percent = Mass of Solute / Total Mass of Solution.
Flash Cards
Glossary
- Mass Percentage
A way to express the concentration of a solute in a solution, indicating the mass of a component relative to the total solution mass, expressed as a percentage.
- Mass of Solute
The weight of the solute present in the solution.
- Total Mass of Solution
The combined mass of the solute and the solvent in a solution.
Reference links
Supplementary resources to enhance your learning experience.
