Sample Loop in Gas Chromatography
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Thermal Desorption
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll learn about thermal desorption in gas chromatography. Can anyone tell me what they think thermal desorption might be?

Isn't it the process where heat is used to release compounds from a solid?

Exactly, Student_1! Thermal desorption uses heat to rapidly release volatile compounds. This is crucial for ensuring that the sample injection resembles a pulse to allow accurate measurement and analysis.

So, why is it important to release everything all at once?

Great question, Student_2! When we release all components at once, it reduces the sample volume, making it easier for GC to analyze. Think of it like launching a burst of fireworks instead of letting them off individually!

How do we ensure that we are releasing all the volatile compounds efficiently?

We use 'flash desorption,' where the temperature increase is rapid. This method ensures all volatile compounds are released quickly—key for effective chromatography.

What are volatile organic compounds exactly?

VOCs are compounds with high vapor pressure at room temperature, often detrimental to health and the environment. We need precise methods to analyze them!

Let’s summarize thermal desorption: it releases samples rapidly using heat, enabling accurate analysis of VOCs and ensuring minimal loss.
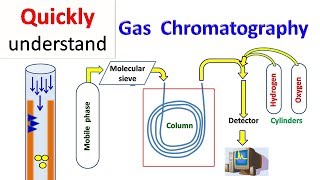
Sample Loop Functionality
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on to the sample loop, why do you think it’s important in gas chromatography?

Is it like a holding area for the sample before it goes into the GC?

Exactly, Student_1! The sample loop holds the sample and allows for precise measurements of concentration. It’s crucial for calibrating results in the GC.

How do we make sure that the sample loop is properly calibrated?

Calibration involves injecting known concentrations into the GC and ensuring the sample loop holds the same volume. This guarantees that what we measure corresponds accurately.

What happens if the calibration is off?

If it’s incorrect, all our results will be flawed, leading to potential environmental risks. That’s why maintaining calibration is so essential!

So, are there specific volumes we use for the sample loop?

Yes, the volume can vary significantly, depending on the application—ranging from 100 microliters to 1 milliliter. This variability helps fit different experiment needs.

In summary, the sample loop holds and calibrates the sample for accurate GC analysis, ensuring we understand what pollutants we’re measuring!
Purging and Trapping Techniques
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Another method we use is purging and trapping. Who can explain how it works?

I think it involves using gas to get VOCs out of the water, right?

Correct, Student_1! Nitrogen gas is often used to purge the volatile compounds from water, effectively stripping these compounds for analysis.

Then what happens to the collected vapors?

Once purged, these vapors are collected in a thermal desorption tube, just like in our previous discussions. They then get analyzed using the GC.

Is this method better than traditional liquid-liquid extraction?

Absolutely! It minimizes the risks of losing volatile compounds that can occur with traditional methods. Plus, it’s faster!

So, purging traps the VOCs until they are ready for analysis?

Exactly! This method helps in ensuring we collect accurate samples for environmental monitoring.

To recap: purging and trapping efficiently extracts VOCs from water, providing a fast and reliable way to analyze samples.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The text explains the method of thermal desorption and emphasizes its importance in gas chromatography (GC). It describes how a sample loop functions to accurately measure sample volume and concentration. The use of automated thermal desorption units aids in the quick and efficient analysis of volatile compounds, which is critical for environmental monitoring.
Detailed
Sample Loop in Gas Chromatography
This section covers the process of thermal desorption and its integration in gas chromatography (GC), providing insights into the significance of sample loops.
Key Concepts:
- Thermal Desorption: A method where samples are heated to release absorbed analytes quickly, known as flash desorption. This technique aims to inject samples in pulse form for effective chromatographic analysis.
- Sample Loop: An essential component in a gas chromatograph that holds the sample before it is entered into the GC. Proper calibration of the sample loop is crucial for accurate measurements. The volume and concentration of the sample must be precisely known to ensure reliable chromatographic results.
Importance in Environmental Monitoring:
- This methodology is particularly pertinent for analyzing volatile organic compounds (VOCs) due to their high vapor pressure and volatility. The text illustrates the benefits of using automation to minimize manual handling and reduce potential sample loss during analysis.
- The section also touches upon related processes, such as purge and trap techniques for water sampling, further underlining the technological advancements in monitoring environmental quality.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Sample Loop in GC
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
GChas what is called as a sample loop. The sample loop is the holding place for the sample, you are sending in certain concentration. And you would like the sample loop to hold all the sample that you are bringing. This is important because what you measure in the GC depends on what you are injecting and what you are desorbing.
Detailed Explanation
The sample loop in gas chromatography (GC) acts as a container that holds the sample to be injected into the analytical instrument. This is crucial because accurate, consistent measurements depend on knowing the exact amount of the sample being used. If the sample loop does its job well, it ensures that the measurements taken by the GC are reliable and reproducible.
Examples & Analogies
Think of the sample loop like a measuring cup in cooking. Just like you need a precise amount of an ingredient to bake a cake, the GC needs to know the exact amount of a sample to measure it accurately. If you were to use too much or too little, the cake wouldn’t rise correctly, just like inaccurate sample measurements can lead to incorrect analysis results.
Functionality of the Sample Loop
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It has to be in a finite well-defined volume so that you know what that volume is, you know what the sample volume is, otherwise you don’t know what you are injecting what is coming out and all this is a big mess.
Detailed Explanation
The sample loop must have a defined volume to ensure that the amount of gas being analyzed is known. This allows chemists to relate the measurement results to the concentration of substances in the original sample. Without this known volume, it would be difficult to replicate experiments or compare results accurately, leading to potential confusion and errors.
Examples & Analogies
Imagine pouring orange juice into a glass that has measurement markings. If you don’t know how much juice you poured, you can’t say how strong or weak your orange juice is. In the same way, if the sample loop doesn’t have a defined volume, the GC can’t determine the concentration of components in the gas sample.
Calibration and Sample Volume
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, this is all carefully calibrated and done. So the sample loop will allow you to keep this in place, ok, let’s not get into sample loop, you can go and read about it, you can type GC sample loops and the schematics for that comes, very neat system.
Detailed Explanation
Calibration of the sample loop ensures that the device accurately measures the sample it holds. This calibration process makes it possible to confirm that the measurements taken by the GC relate directly to the actual concentrations of the substances being analyzed. Various factors like temperature and pressure must be considered during calibration.
Examples & Analogies
If you have a scale to weigh ingredients, you need to calibrate it often to make sure it gives accurate readings. Just like this, a sample loop must be calibrated to ensure the GC can provide the correct analysis of the substances within the gas sample.
Importance of Sample Injection Timing
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Otherwise, separations will have a problem in sample injection, your sample chromatography, retention time will change and all that so, it is very difficult to manage it.
Detailed Explanation
The timing of sample injections into the GC is critical for the separation process, which is essential for effective analysis. If the injection isn’t managed properly, it can affect the retention time of components in the chromatography, making it challenging to achieve precise results. Consistency in injection helps maintain predictable outcomes.
Examples & Analogies
Think about a race where all cars must start at the same moment to compete fairly. If one car starts later, it throws off the entire race outcomes. Similarly, if the sample injection in a GC doesn’t happen at the correct time, it can disrupt the analysis, leading to confusion regarding the results.
Key Concepts
-
Thermal Desorption: A method where samples are heated to release absorbed analytes quickly, known as flash desorption. This technique aims to inject samples in pulse form for effective chromatographic analysis.
-
Sample Loop: An essential component in a gas chromatograph that holds the sample before it is entered into the GC. Proper calibration of the sample loop is crucial for accurate measurements. The volume and concentration of the sample must be precisely known to ensure reliable chromatographic results.
-
Importance in Environmental Monitoring:
-
This methodology is particularly pertinent for analyzing volatile organic compounds (VOCs) due to their high vapor pressure and volatility. The text illustrates the benefits of using automation to minimize manual handling and reduce potential sample loss during analysis.
-
The section also touches upon related processes, such as purge and trap techniques for water sampling, further underlining the technological advancements in monitoring environmental quality.
Examples & Applications
Using thermal desorption to analyze benzene concentrations in water samples.
The application of a sample loop for accurately measuring known concentrations of a gas before analysis.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Heating up the samples, oh so quick, thermal desorption is the trick!
Stories
Imagine a scientist in a hurry, heating samples to analyze the flurry of VOCs in the air—each second counts, so she uses thermal desorption for results that mount!
Memory Tools
To remember VOCs—Vapor On the Chart—these compounds need rapid release!
Acronyms
GC
Gas Chromatography - where Good Chemistry happens through precise calibration.
Flash Cards
Glossary
- Thermal Desorption
A method of releasing compounds from solid or liquid matrices using heat.
- Sample Loop
A component in gas chromatography that holds a specific volume of sample for injection into the system.
- Volatile Organic Compounds (VOCs)
Compounds with high vapor pressure at room temperature, often found in household and industrial products.
- Calibration
The process of configuring an instrument to provide a result for a sample within an acceptable range.
- Purge and Trap
A method for extracting volatile contaminants from liquid samples using an inert gas.
Reference links
Supplementary resources to enhance your learning experience.
