Composition and Analysis of Particulate Matter
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Measurement Techniques for PM
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss the different techniques used for measuring particulate matter or PM. Can anyone tell me what gravimetric measurement is?

Isn't that when we collect particles on a filter and weigh them?

Exactly! That's a fundamental method. Now, can you think of any other measurement techniques?

What about optical methods that use lasers?

Yes! Optical methods like the Aerodynamic Particle Sizer (APS) measure particle size using laser scattering. Remember, different methods may give different results for the same PM. This helps us understand the size distribution in the air.

Why do the results differ?

Great question! It's due to the differences in how particle sizes are defined in each method, such as optical and aerodynamic diameters. They’re not necessarily the same, so calibration is vital.

To remember this difference, think of the acronym C.O.A.T — Calibration, Optical, Aerodynamic, and Technique!

Got it! Calibration ensures accurate comparisons, right?

Yes! Now, let's summarize: We discussed gravimetric measurements and optical methods like APS. Both methods provide different insights about PM, and calibration with standard particles is crucial for accurate analysis.
Role of Standards in PM Measurement
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive deeper into the importance of using standards in PM measurement. Can anyone explain what we mean by standard particles?

Aren't they particles with known size and density?

Correct! When we calibrate instruments using these standard particles, it allows us to correlate results from different measurement techniques. What happens if we don't use standards?

The measurements could be all over the place and not comparable?

Exactly! That’s why we need to establish clear correlations and procedures. Keep in mind the concept of 1 gram density for determining the aerodynamic diameter.

How do we find out if our measurements are accurate?

By conducting experiments with the same particles across instruments to see if their size distributions match. This consistent verification is crucial for our data validity.

To remember this, you can say: Standards are the backbone of measurement accuracy. Just as a good foundation supports a building!

So without them, our findings might lead to incorrect conclusions?

Exactly! So far, we’ve covered standard particles and their pivotal role in calibration. Moving forward, we will discuss the composition of the filter media.
Filter Media Composition
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s focus on the filter media used for collecting PM samples. What do you think makes the choice of filter so essential?

Could it affect how much we collect and analyze?

Absolutely! If the filter doesn’t trap the particles effectively, we lose valuable data. The choice also impacts whether we can analyze organic versus inorganic components. Can anyone give an example of a preferred filter type and its purpose?

I remember quartz fiber filters are good for organic analysis because they don’t leach materials.

Exactly! Quartz fiber can withstand high temperatures, making it ideal when analyzing organics. What about when we need to measure metals?

Maybe Teflon filters since they are inert?

Yes, Teflon filters are preferred for metals analysis as they don’t interact with solvents. Remember: Filter choice is crucial – think of it like choosing the right tool for the job!

If I want to keep it simple, just remember: Quartz is for organics; Teflon is for metals.

Well done! Let’s summarize today’s discussion: We explored the importance of filter media choice based on target analysis and its influence on measurement accuracy.
Analysis of Organic and Inorganic Components
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s talk about analyzing the composition of PM. What are the two main categories of particulate matter?

Organic and inorganic!

Correct! For organic components, we often use instruments like Gas Chromatography (GC). What’s the challenge with analyzing pure carbon?

It doesn't dissolve in solvents so it’s hard to analyze, right?

Exactly! That's why we distinguish organic carbon from elemental carbon. What about inorganic analysis? How do we typically approach that?

I've heard of methods like ICP for elemental analysis?

Good point! We use methods like ICP and ion chromatography for ions. It’s essential to separate these components for a complete understanding of PM.

To remember: OC and EC for organs, ICP and ion chromatography for inorganics. Let's summarize: We covered the methods to analyze organic and inorganic PM components and discussed specific challenges.
Practical Implications of PM Analysis
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s bring all this together: why does analyzing PM composition matter in real life?

It helps in air quality management and policy making!

Great insight! Understanding PM helps us control sources of pollution effectively. How does the filter method we choose affect policy decisions?

If data is not accurate, it can lead to poor regulations or decisions!

Precisely! The integrity of analysis impacts public health and environmental policies significantly. To summarize, we've explored the wider implications of PM analysis in terms of environmental policy and health.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section highlights the different techniques for monitoring particulate matter in the air, including gravimetric measurements and optical methods. It emphasizes the need for careful calibration using standard particles to ensure valid comparisons between different measurement techniques. Additionally, it discusses the significance of filter media composition, particle analysis, and the extraction methods necessary for evaluating organic and inorganic components.
Detailed
Composition and Analysis of Particulate Matter
This section elaborates on the crucial concepts of measuring and analyzing particulate matter (PM) in the air. It distinguishes between various measurement techniques, such as gravimetric measurements and optical methods, including aerodynamic particle sizing and electrical mobility measurements.
Key Techniques for PM Measurement
- Gravimetric Measurements: Involves collecting particles on filter media to assess mass directly.
- Optical Methods: These methods involve using lasers to measure particle size through scattering and involve instruments like the Aerodynamic Particle Sizer (APS).
- Electrical Mobility Diameter (DMA): Compares different diameters based on mobility; understanding that diameter measurements can differ across methodologies is critical.
Calibration and Standards
Accurate comparisons between different measurement techniques require calibration with standard particles, which are defined as spherical particles with known size and density. The aerodynamic diameter is established based on the mobility of these standard particles at a density of 1 gram per cubic centimeter.
The relationship between different diameters requires carefully planned experiments to establish correlations, especially regarding PM 10 measurements that may vary across methods.
Importance of Filter Media Composition
The choice of filter media is essential, as it affects both mass measurement and composition analysis of collected particulate matter. Different filters are used based on the target analysis (organic, inorganic, metals) and influence extraction methods. Various solvents are discussed for extracting components from PM, as well as their implications for accuracy.
Composition Analysis
Particulate matter can be categorized as either organic or inorganic. Techniques such as GC for organic analysis and ICP for elemental analysis are utilized to determine the composition of PM effectively.
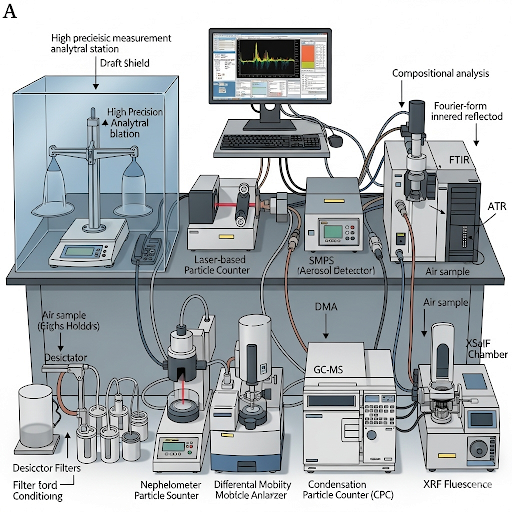
In conclusion, the intricate balance between accurate methods of sampling, analysis, and understanding the environmental implications of particulate matter is essential for effective monitoring and policymaking.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Measurement Methods for Particulate Matter
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So we will continue from where we left yesterday we are looking at the analysis of PM. We looked at different methods of measurement, quantification for the entire range. So, we were looking at gravimetric measurements which involved a collection on a filter media versus other techniques which are things like optical techniques.
Detailed Explanation
This chunk introduces the different methods for measuring particulate matter (PM). The gravimetric method collects PM on a filter to measure its mass, while optical techniques use light to analyze particle size. Gravimetric measurements provide direct mass data, which is fundamental for understanding air quality.
Examples & Analogies
Imagine weighing the dirt from your shoes after walking outside. Gravimetric measurement is like this: it tells you how much dirt (PM) was collected. On the other hand, optical techniques are like using a camera that shows you the size and number of particles without needing to weigh them directly.
Understanding Different Diameter Measurement Techniques
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We also looked at something called as electrical mobility which is a DMA. The electrical mobility diameter is different sizes at different phases. So, all these 3 are not the same. So, if you want to compare these 3 data sets you have to do an experiment with the same kind of particle.
Detailed Explanation
In this chunk, different methods of measuring particle size—gravitational, optical, and electrical mobility—are discussed. Each method provides a different type of diameter measurement that can vary based on the particle's properties. To effectively compare results from these methods, similar particles must be tested under the same conditions.
Examples & Analogies
Think of measuring the height of a student in different ways: using a ruler (gravitational), a measuring tape (optical), and estimating by eye (electrical mobility). Each method might give a slightly different result, and to be truly accurate, you’d need to measure the same student with each method to see how they compare.
Calibration and Standards for Measurement
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, again as with any other analytical method that we are looking at, we need standards. We are looking at optical diameter versus particle aerodynamic diameter, I mean we need standards here are standard particles.
Detailed Explanation
This section emphasizes the need for calibration and the use of standard particles which have known sizes and densities. Standards help ensure that different measuring instruments provide comparable and accurate results when analyzing particulate matter.
Examples & Analogies
Imagine baking bread; to ensure it rises properly, you must use the right amount of yeast and flour. In the same way, using standards in particulate measurement ensures that you get the 'right' size readings for your particles, allowing for reliable results.
Advantages and Limitations of Filter Methods
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, we are also talking about the advantages of using a filter, the purpose of using filters one is composition, one of the main things is composition.
Detailed Explanation
This chunk explains the significance of using filters for collecting particulate matter. Filters can provide not only the mass of particles but also important information about their chemical composition. However, there are some limitations such as difficulty in analyzing very small particles and contamination risks during sampling.
Examples & Analogies
Consider using a tea strainer when brewing tea. The strainer allows you to collect tea leaves while letting the liquid pass through. Similarly, filters are used to catch particulate matter and analyze its composition, though one must ensure that no leaves escape or get into your drink.
Techniques for Analyzing Particulate Composition
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, we want to do the composition of PM, you have 2 options again you can look at organic or inorganic.
Detailed Explanation
Here, the text points out the two main categories for analyzing the composition of particulate matter: organic and inorganic components. Organic matter might involve compounds with carbon, while inorganic includes elements like metals and salts. This differentiation helps in understanding sources and effects of PM in the environment.
Examples & Analogies
Think of your grocery shopping list. You might categorize your items into 'fruits and vegetables' (organic) and 'canned goods and cleaning supplies' (inorganic). Similarly, breaking down particulate matter into organic and inorganic helps scientists track pollution sources more effectively.
Methods for Organic and Inorganic Analysis
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For the organic analysis, we do what is called as OC, EC analysis is called organic carbon, elemental carbon.
Detailed Explanation
This chunk details specific methods for organic and inorganic analysis of particulate matter. Organic Carbon (OC) refers to carbon in organic compounds, while Elemental Carbon (EC) refers to pure carbon forms like soot. Knowing the proportion of these types can reveal the pollution source, such as vehicle emissions.
Examples & Analogies
Consider a classroom filled with students where some are wearing uniforms (elemental carbon representing a specific source like soot) and others are dressed casually (organic carbon representing various other sources). By identifying and quantifying each group, you can tell which students are associated with which activities, similar to tracing pollution sources by analyzing carbon types.
Key Concepts
-
Different measurement techniques for PM such as gravimetric and optical methods.
-
Importance of using standard particles for calibration in measurements.
-
Significance of filter media in accurately measuring and analyzing PM.
-
Methods of analyzing organic vs inorganic PM components.
Examples & Applications
Using a glass fiber filter for PM mass measurement due to its ability to capture particles effectively.
Employing Gas Chromatography for analyzing organic compounds in particulate matter.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When measuring PM for the air, gather particles with utmost care. Filters trap, weigh it right, ensuring data shines so bright.
Stories
Once, in a lab, a scientist named Dr. Particulate discovered that using the right filter was like choosing the best fishing net - it catches just what you want!
Memory Tools
Remember OAE for analysis: Optical for size, Aerodynamic for drag, and Electrical for mobility!
Acronyms
C.O.A.T
Calibration
Optical
Aerodynamic
Technique - to remember key concepts in PM measurement.
Flash Cards
Glossary
- Particulate Matter (PM)
A mixture of solid particles and liquid droplets found in the air.
- Gravimetric Measurement
The method of measuring the mass of particles collected on a filter.
- Optical Diameter
The size of a particle measured using optical techniques.
- Aerodynamic Diameter
The diameter of a particle that has the same aerodynamic drag force as a sphere of a specified diameter.
- Electrical Mobility Diameter
The diameter associated with a particle's drift velocity in an electric field.
- Standard Particulate
Particles of known size and density used for calibration.
- Organic Carbon (OC)
Carbon derived from organic compounds, typically containing hydrogen, oxygen, and nitrogen.
- Elemental Carbon (EC)
Carbon in its elemental form, often produced by combustion, also known as soot.
- Ion Chromatography
A method for separating and analyzing ions in solution.
Reference links
Supplementary resources to enhance your learning experience.
