Organic and Elemental Carbon Analysis
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Carbon Analysis
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll be discussing the importance of analyzing organic and elemental carbon in particulate matter. Can anyone explain why carbon analysis is crucial in environmental science?

It helps us understand air pollution and its effects on health and the environment.

Exactly! Monitoring carbon levels helps us develop policies to improve air quality. Now, what do you think are the two primary forms of carbon we analyze?

Organic carbon and elemental carbon, right?

Correct! Organic carbon refers to carbon in various compounds, while elemental carbon is pure carbon, often found in soot. Remember the acronym OES for 'Organic and Elemental Carbon Analysis'! Let's move on to how we measure these.
Measurement Techniques
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What methods do we use to measure organic and elemental carbon, and what is their significance?

We can use gas chromatography for organic carbon. It's essential for understanding pollution sources.

Good point! Gas chromatography is critical for analyzing organic compounds. Now, can someone explain the difference in extraction techniques for organic and elemental carbon?

For organic carbon, we use organic solvents, and for elemental carbon, we often need acids like nitric acid.

Exactly! Just remember—solvents for organic and acids for elemental carbon. A helpful mnemonic is SOIL: Solvents for Organic, Acids for Elemental. Let's explore the implications of these analyses.
Challenges and Importance of Standardization
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Why do you think standardization is important in carbon analysis?

To ensure that we can compare results across different studies accurately.

Exactly! Standard particles help us to calibrate and rectify methods. What challenges do you think might arise if we don’t use standardized techniques?

We could get unreliable data, which might lead to incorrect policy decisions.

Precisely! Lack of standardization can distort findings. Remember, data integrity is essential. Let’s discuss how data from carbon analysis can influence environmental policies next.
Real-World Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How does analyzing organic and elemental carbon contribute to environmental policy-making?

It helps identify pollution sources, so regulations can target those specific industries.

Exactly right! Data on carbon levels can lead to regulations that reduce emissions significantly. Can you think of any recent policies that might have been influenced by such data?

Maybe the Clean Air Act? It targets pollutants based on studies.

Great example! The Clean Air Act is indeed influenced by extensive air quality data. Always remember, informed policies come from solid data. Let’s sum up.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides an in-depth look at the analysis of organic and elemental carbon in environmental samples, detailing the significance of accurate measurement techniques such as gas chromatography (GC) and combustion analysis. It stresses the necessity of using suitable solvents for extraction, the difference between organic carbon and elemental carbon, and the importance of standardization in obtaining reliable data.
Detailed
Overview
This section focuses on the analytical techniques for measuring organic and elemental carbon in particulate matter (PM). Understanding the composition of PM is crucial due to its environmental and health impacts.
Key Points
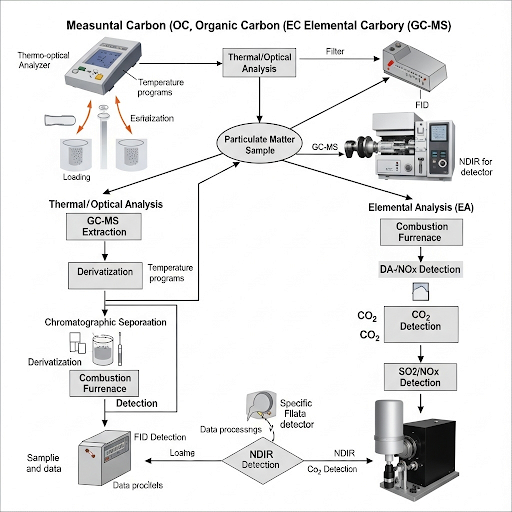
- Instrumentation: Techniques such as gas chromatography (GC) and elemental analysis are utilized for identifying and quantifying organic and elemental carbon in PM.
- Organic Carbon (OC) includes carbon that is part of various organic compounds found in the atmosphere, while Elemental Carbon (EC) refers to pure carbon often in the form of soot.
- Extraction Techniques: Different solvents are required to extract compounds from particulate matter effectively.
- Organic solvents (e.g., hexane, dichloromethane) are used for organic extraction.
- For metals and elemental carbon, acids like nitric acid (HNO₃) are commonly used, although they don’t dissolve silicon.
- Importance of Standardization: To compare results across different measurement methods, standardized particles of known size and density must be used.
- Environmental Implications: The results from carbon analyses provide essential data that inform environmental policies and regulations aimed at improving air quality.
- Challenges in Analysis: Variations in filter compositions and extraction methods affect the accuracy of measurements, necessitating careful method selection to reduce leaching and contamination of samples.
Conclusion
Accurate analysis of organic and elemental carbon in PM is critical for understanding air quality and its health implications. By employing specific techniques and careful standardization, researchers can acquire valuable data to inform environmental management strategies.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Organic and Elemental Carbon Analysis
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So we want to do the composition of PM, you have 2 options again you can look at organic or inorganic. So we are looking at organic you know the instruments that are used for organic we saw that already in terms of the water, if you want to look at organic chemicals, there are a set of instrumentation that you can use starting from the GC or LC or any of those instrumentation.
Detailed Explanation
In this chunk, we introduce the two main categories of carbon analysis: organic and inorganic. The focus is on organic analysis, wherein various instruments such as Gas Chromatography (GC) or Liquid Chromatography (LC) are employed to study organic compounds. Organic carbon analysis is essential because it helps identify different carbon compounds present in particulate matter (PM) and their sources.
Examples & Analogies
Think of organic carbon analysis like examining a soup's ingredients. Just as you identify each ingredient (like vegetables, meat, or spices) to understand the soup better, organic analysis allows scientists to identify different organic compounds to understand their presence in air particulate matter.
Methods for Analyzing Particulate Matter
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, let us say a particle is there, this particle itself may be an organic particle, which means that you have to dissolve the entire particle into a solvent. So, that is one option. Second option is if the organic material is adsorbed on other particles and when you have to pull these particles out, you have to dissolve or desorb.
Detailed Explanation
This chunk discusses the two primary methods for analyzing organic particles in particulate matter (PM). One approach is to dissolve the entire particle in a suitable solvent to extract its components. The second method involves desorbing organic materials that are attached to other particles. This distinction is crucial since the chemical nature of the particles can significantly affect the analysis.
Examples & Analogies
Imagine trying to retrieve a chocolate chip from a cookie. You could either dissolve the entire cookie in hot milk (dissolution) to get the chocolate or gently scrape it off with a knife (desorption). In our analysis, we must choose the appropriate method based on how the organic materials are associated with the particles.
Understanding Organic Carbon and Elemental Carbon
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Here again there is a distinction. So, if you want to pull out look at composition of some of these things, if they are adsorbed on something else, they will nicely show up in something like the GC or LC but if it is pure carbon, it may not show up there because it will not dissolve in anything, it will sit there and it is elemental.
Detailed Explanation
In this segment, we differentiate between organic carbon and elemental carbon. Organic carbon includes compounds comprised of carbon along with other elements such as hydrogen and oxygen, while elemental carbon refers strictly to carbon in its pure form. This distinction is important because while organic carbon can often be analyzed using standard methods, elemental carbon may need alternative techniques since it does not dissolve in solvents like organic carbon does.
Examples & Analogies
Consider a fruit salad (organic carbon) versus sugar (elemental carbon). While you can easily identify and taste each fruit in the salad using a fork, trying to analyze sugar might require a different method, like dissolving it in water to understand its properties, which is not needed for the fruit salad.
Techniques for Elemental and Inorganic Analysis
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The other kind of analysis that you have is inorganic analysis. So, inorganic analysis, you can do 2 kind of things you can do elemental analysis or you can do ions, you can get what is present on the particle by either of these methods.
Detailed Explanation
This chunk introduces inorganic analysis, which can either focus on elemental analysis (e.g., identifying specific metal concentrations) or ion analysis (e.g., detecting specific ions in the particulate matter). Both methods provide valuable information about the composition of particles in the air and can inform pollution sources and impacts.
Examples & Analogies
Think of inorganic analysis as checking the nutritional content of a food item. Elemental analysis is like checking how much protein or fat is there, while ion analysis is akin to inspecting for specific vitamins or minerals. Each type of analysis sheds light on different aspects of the nutrients in the food.
Extraction and Challenges in Analysis
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
What solvent can we use and how? An acid, which is the acid that has capacity to dissolve everything, HNO3, so all nitrates are supposed to be soluble.
Detailed Explanation
This section explores the extraction process for metals in particulate matter analysis. Concentrated nitric acid (HNO3) is often used as a solvent because it effectively dissolves various metals from PM. However, special care must be taken since extracting requires considering the material of the filter used to collect the samples and potential interferences that might arise during the analysis.
Examples & Analogies
Imagine cleaning an old metal pot with vinegar (acid) to remove rust. Just like vinegar helps dissolve the rust, nitric acid helps dissolve certain metals in particulate samples. However, you must be careful not to use the wrong cleaning materials that could scratch or damage the pot.
Practical Considerations for Sampling
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, you have at least 3 filters. One for each and they are running concurrently, which means that you need to have 3 EPM sampler or 3 of these sampler running concurrently.
Detailed Explanation
This chunk discusses the practical needs for collecting air samples for analysis. It highlights that at least three different filters are necessary to separately capture organic carbon, elemental carbon, and metal content. The need for concurrent sampling ensures accurate data collection across different analyses, allowing for comprehensive profiling of the particulate matter.
Examples & Analogies
Think of this as preparing a party where you need to set up three different tables for appetizers, main courses, and desserts. Each table serves a different purpose, ensuring your guests get a complete dining experience. Similarly, using separate filters ensures that each type of carbon or metal is accurately captured for analysis.
Key Concepts
-
Carbon Analysis: The study of carbon in environmental samples to assess air quality.
-
Organic vs Elemental Carbon: Differentiates between carbon in compounds and pure carbon forms.
-
Extraction Methods: Specific techniques used to isolate organic and elemental carbon.
-
Standardization: The need for uniform measurement techniques across studies.
Examples & Applications
A study analyzing urban air samples to determine the source of particulate pollution.
Use of gas chromatography to identify specific organic compounds in smoke from wildfires.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
OC is in the living spree, EC in soot you can see.
Stories
Think of a factory where smoke rises high; the smoke contains EC, while the green fields below represent OC from plants.
Memory Tools
SOIL = Solvents for Organic, Acids for Elemental.
Acronyms
OC/EC = Ongoing Care for environment / Essential Carbon.
Flash Cards
Glossary
- Organic Carbon (OC)
Carbon found in organic compounds such as hydrocarbons, which can come from living organisms or fossil fuels.
- Elemental Carbon (EC)
Pure carbon in the form of soot, often a product of incomplete combustion.
- Gas Chromatography (GC)
An analytical method used to separate and analyze compounds that can be vaporized.
- Standard Particles
Particles of known size and density used for calibration in analytical instruments.
- Extraction Techniques
Methods used to isolate compounds from a mixture, often involving solvents or acids.
Reference links
Supplementary resources to enhance your learning experience.
