Examples of Environmental Systems
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Transport Mechanisms
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're diving into mass transport mechanisms. Can anyone tell me what molecular diffusion is?

Isn't it when molecules move from a high concentration area to a low concentration area?

That's correct, Student_1! It's key in understanding how pollutants spread in still waters. Now, how does this differ from bulk flow?

Bulk flow happens when there’s actual movement of the fluid, right?

Exactly, Student_2! Do you remember what we might call bulk flow sometimes?

Oh, 'advection'!

Great recall, Student_3! Remember: diffusion is slow and happens in stagnant environments while bulk flow quickly moves concentrations. Let's move on to examples in real-world systems.
Environmental Systems: Lakes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's examine lakes. What can affect mass transfer in deep lakes?

Wind can create surface currents!

Exactly! And how does that impact diffusion in deeper layers?

In the deeper parts, diffusion is important because there's not much mixing.

That's right! So, when we're looking at chemical concentrations in lakes, we need to consider both diffusion and advection. Can anyone think of a scenario where diffusion is the primary transport mode?

Maybe in a lake during winter when the surface is colder?

Excellent thought, Student_2! Seasonal changes really influence these dynamics. Remember that diffusion continues even in still areas.
Rivers and Movement of Pollutants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s move on to rivers. Why do we say that molecular diffusion is less significant in rivers?

Because of faster flow rates!

Exactly! Here, advection dominates. It's often the principal mechanism of transporting pollutants down the river. What implications does this have on pollution mitigation?

It means we need to worry more about where pollutants enter the river!

Absolutely right! Spotting pollutant entry points is crucial for effective monitoring and regulation. Let’s summarize what we’ve learned...
Groundwater and Pollutant Transport
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss groundwater now. What makes transport here different compared to rivers and lakes?

The flow is really slow in groundwater!

Correct! What's the main mechanism driving transport in many groundwater scenarios?

I think it's diffusion, especially in unsaturated zones where there's not much water.

Exactly! Recall that in areas with little moisture, diffusion can dominate. How do geological factors impact this process?

Yeah, different soils will allow or restrict diffusion, right?

Spot on, Student_3! Understanding soil types helps us grasp diffusion rates and contaminant transport in groundwater.
Conclusions and Recap
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we conclude our exploration of environmental transport mechanisms, can anyone summarize the lessons learned about mass transfer in these systems?

Mass transfer involves diffusion and bulk flow. In rivers, bulk flow dominates, while in lakes and groundwater, both mechanisms can play unique roles.

And for lakes, seasonal changes can shift how these processes operate!

Great job, everyone! Remember, understanding these interactions is vital for environmental protection and management. Keep these concepts in mind as you study further!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section presents an interaction between molecular diffusion and bulk flow in different environmental contexts like lakes, rivers, and groundwater systems. It highlights unique scenarios where diffusion predominates, including the role of temperature gradients and geological factors affecting particle movement in these environments.
Detailed
Examples of Environmental Systems
This section explores several key environmental systems, including lakes, rivers, oceans, and groundwater, focusing primarily on the mechanisms of mass transfer—molecular diffusion and bulk flow (or advection).
Mass Transport Mechanisms
- Molecular Diffusion: This is the process by which molecules move from areas of high concentration to low concentration without bulk movement. It plays a vital role in pollutants' movement in stagnant environments like lakes.
- Bulk Flow / Advection: This refers to the movement of concentrations due to flow velocity. In many instances, bulk flow dominates, overshadowing molecular diffusion.
Environmental Examples
- Lakes: In deep lakes, wind-induced circulation can create well-mixed upper layers while deeper areas may rely on diffusion for mass transfer, particularly if thermal convex gradients exist. This variability can depend on seasonal changes and the physical characteristics of the lake.
- Rivers: Here, flow is significantly more rapid, making bulk flow the primary mode of mass transfer, with molecular diffusion being relatively negligible.
- Oceans: Similar to lakes, ocean currents caused by wind and density differences also facilitate pollutant transport, operating on larger scales.
- Groundwater: Groundwater movement is slow, influenced by geological variations. In some contexts, diffusion becomes the predominant transport mechanism, particularly in unsaturated zones where air movement is minimal.
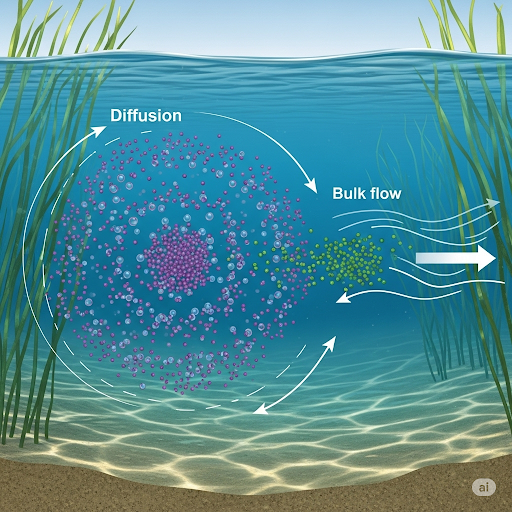
The understanding of these phenomena is critical for environmental monitoring and analysis, offering insights into pollutant behavior and transport mechanisms.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Mass Transfer in Environmental Systems
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, in systems like rivers where there is a flow, the flow is significant, this is most likely true. The second case is most likely true. Is there any system that the first one is true? Any environmental system where the first one, where the advection or bulk flow is very small, the diffusion is what primarily drives mass transfer? Can you think of any environmental system?
Detailed Explanation
In various environmental systems, mass transfer can occur through two main mechanisms: molecular diffusion and advection (bulk flow). In rivers, there is usually significant flow, meaning advection dominates the mass transfer process. In contrast, in certain scenarios, particularly where flow is minimal or negligible, diffusion becomes the primary mode of mass transfer. Understanding these mechanisms helps in predicting pollutant transport and chemical distribution in different environments.
Examples & Analogies
Consider two different bodies of water: a fast-moving river and a still pond. In the river, if you drop a coloring agent, it will spread rapidly due to the swift current (advection). In the still pond, however, the same agent would spread slowly, primarily by diffusion, illustrating how the flow (or lack thereof) affects the transport of substances.
Role of Diffusion in Lakes
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In a lake, okay. We will come back to that in a minute. Any other system? Can there be bulk flow in lake? I think we discussed this long back. So there is advection because of wind, wind induced circulation can happen and thermal convection can occur.
Detailed Explanation
Lakes can exhibit both bulk flow and diffusion. When wind blows across the lake surface, it initiates a circulation of water, which is known as advection. Additionally, thermal convection can occur when temperature differences in the water lead to variations in density, causing water to mix. Thus, in lakes, depending on their depth and environmental conditions, both diffusion and bulk flow can significantly influence the distribution of chemicals.
Examples & Analogies
Imagine a large lake on a windy day. As the wind pushes across the water, the surface layers move, mixing with deeper layers. Now, think of adding a drop of food coloring on one side: it will not just sit there; the wind-induced waves will help disperse it across the lake faster than if it were calm. This analogy illustrates how wind contributes to mixing in lakes, while diffusion still plays a role in the deeper, still parts of the water.
Groundwater Flow and Diffusion
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Groundwater essentially means it is soil, we are talking about soil systems. So, soil systems, there is a water table and there is an aquifer, these are porous media.
Detailed Explanation
Groundwater flows through the soil, comprising both saturated and unsaturated zones. The movement of groundwater is generally very slow due to the porous nature of the soil, which creates resistance. In these cases, both diffusion and flow due to pressure differences are essential for understanding how pollutants are transported within the groundwater system. Since the velocity of groundwater is much slower compared to rivers, diffusion can also play a significant role.
Examples & Analogies
Consider a sponge soaked in water and then placed on a flat surface. The water can move out slowly through diffusion. Similarly, in the soil, when there’s a pollutant, it slowly spreads out in the groundwater, much like how the water spreads out on the surface due to capillary action. This slow movement emphasizes how diffusion can dominate in such environments compared to faster systems like rivers.
Unsaturated Zone and Capillary Action
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, all this is capillary action. The groundwater level is after capillary action, it has risen to some distance and it will stop, it will not keep going.
Detailed Explanation
In the unsaturated zone above the groundwater table, water is not fully saturating the soil, and capillary action helps in moving moisture upward to some extent. This capillary action can enable contaminants to move through the unsaturated soil layer primarily by diffusion. When analyzers look for pollutants in this zone, they often focus on how these contaminants diffuse into the air or deeper soil layers where water flow is minimal.
Examples & Analogies
Think of watering a dry sponge. Initially, only the bottom soaks up the water (saturation) while the rest remains dry. However, as water is drawn upward through small pores (capillaries) into the sponge, it slowly spreads out. This is similar to what happens with pollutants in the unsaturated soil where they rely heavily on diffusion, especially when there isn’t enough water to create significant flow.
Transport in Sediments
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, when chemical moves in here, also it is by diffusion predominantly, there is no advection in this case also.
Detailed Explanation
In sediment environments, chemicals typically move through diffusion rather than through advection. This is because sediments are often stationary, and any chemical transport relies on concentration gradients driving the movement of molecules. Understanding this allows for better predictions of pollution over time, as contaminants can remain in the sediment for many years before being released into the water.
Examples & Analogies
Think of old, sedimentary layers at the bottom of a body of water like a historical record. If pollutants were deposited decades ago, over time, they may remain trapped and slowly diffuse into the water as conditions change, similar to how a flavored tea bag slowly releases its taste into water over time. This slow diffusion highlights the patience needed for such pollutants to make their way back into the environment.
Key Concepts
-
Mass Transport Mechanisms: Understanding diffusion and convection in environmental contexts.
-
Lake Dynamics: The impact of seasonal and wind-driven changes on diffusion.
-
River Flow: The predominance of advection in rivers affecting pollutant transport.
-
Groundwater Mechanics: How geological factors and slow flow can affect contaminant diffusion.
Examples & Applications
In a deep lake during winter, diffusive processes can dominate due to thermal gradients, affecting pollutant distribution.
Rivers tend to transport pollutants at a much faster rate due to bulk flow, making diffusion negligible.
Groundwater can have both diffusion and flow, influencing how pollutants spread in soil systems.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a lake so deep and vast, / Wind and warmth mix layers fast. / Diffusion slow, but always there, / In the depths, beware, beware!
Stories
Once upon a snowy winter, a lake stood still. The frosty layer above froze, creating a barrier. Below, a slow-moving current of pollution diffused as the chemicals sought warmth deep beneath, illustrating diffusion in action beneath a frozen surface.
Memory Tools
Remember DIFFUSION vs BULK flow:
Acronyms
Rivers = R.I.V.E.R (Rapid Intrusive Vector of Environmental Risks) highlighting rapid transport implications.
Flash Cards
Glossary
- Molecular Diffusion
The process by which molecules spread from areas of high concentration to areas of low concentration without bulk movement.
- Bulk Flow/Advection
The movement of liquid or gas that carries concentrations along with it, typically associated with velocity.
- Groundwater
Water located beneath the earth's surface in soil pore spaces and fractures of rock formations.
- Thermal Convection
The transfer of heat through the movement of fluids, which can create density-driven fluid flows.
- Stratified Lake
A lake where temperature or other properties create distinct layers resulting in different mixing behaviors.
Reference links
Supplementary resources to enhance your learning experience.
