Fundamentals of Mass Transfer
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Transfer Fundamentals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we'll discuss mass transfer fundamentals, specifically molecular diffusion and bulk flow. Can anyone tell me what mass transfer is?

Isn't it about how substances move from one area to another?

Exactly! Mass transfer involves the movement of species, which can occur through diffusion or advection. Remember, diffusion is the movement driven by concentration gradients.

What about advection?

Advection refers to the transport of substances due to bulk flow, like the movement of water in a river. A good mnemonic to remember is 'ADVection = Air & Water flow'.

Can both happen simultaneously in a system?

Great question! Both can occur. For instance, in rivers, advection often dominates, but diffusion is always happening too. Let's explore scenarios where one predominates over the other.
Scenarios of Mass Transfer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's consider two scenarios: when advection is negligible and when molecular diffusion is insignificant. Can anyone give an example of a system where advection is negligible?

What about lakes?

Correct! In deep, stratified lakes during winter, the top layer cools and mixes due to thermal convection, allowing diffusion to be the primary form of mass transfer in deeper layers. Remember, in winter, diffusion predominates.

So, how does temperature affect this?

Good insight! Temperature gradients influence density and can enhance mixing. Just keep in mind: 'Cooler = denser'! We should also examine how this applies to groundwater situations.
Chemical Transport in Groundwater
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's analyze the movement of chemicals within groundwater. What factors might affect chemical transport in these systems?

Maybe the soil type?

Absolutely! Soil type can determine flow rates due to varying porosity and diffusion rates. More compact soils may hinder flow, making diffusion significant. Think: 'fine soil = slow flow'.

How does that affect pollutant transport?

Pollutants can spread slowly through diffusion in such soils, taking considerable time to observe their effects. This means early actions in pollution control can be crucial. Let's recall our 'slow but steady' mantra!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section outlines the mechanisms of mass transfer, detailing molecular diffusion and bulk flow (advection) in environmental contexts such as lakes and groundwater. It emphasizes scenarios where either mechanism predominates and uses examples to illustrate their interactions in pollutant transport.
Detailed
Fundamentals of Mass Transfer
This section delves into the fundamental concepts of mass transfer, specifically focusing on the dynamics of molecular diffusion and bulk flow in environmental systems. Mass transfer is a critical concept in environmental engineering, influencing pollutant transport and chemical mixing.
Key Points Covered:
- Molecular Diffusion and Bulk Flow: The flux of species A is expressed as a combination of molecular diffusion (denoted as jᵢ) and bulk flow (denoted as uCᵢ). Molecules naturally move from regions of high concentration to low concentration, a process known as diffusion.
- Scenarios of Mass Transfer: The section outlines two primary scenarios:
- When bulk flow is negligible (i.e., uCᵢ ≈ 0), mass transfer primarily occurs due to molecular diffusion.
- When bulk flow is significant (i.e., uCᵢ >> jᵢ), molecular diffusion can often be neglected.
- Environmental Examples: The discussion includes real-world applications, such as the transport of pollutants in different water bodies (lakes, rivers, oceans) and groundwater systems. It highlights situations where diffusion drives mass transfer, such as in deep lakes during winter when thermal stratification occurs.
- Impact of Environmental Factors: Factors such as wind-induced circulation, thermal convection, and sediment interactions are evaluated to illustrate their effects on mass transfer processes.
- Chemical Transport in Groundwater: The importance of both molecular diffusion and advection in groundwater systems is emphasized, considering various geologies and their impact on flow rates.
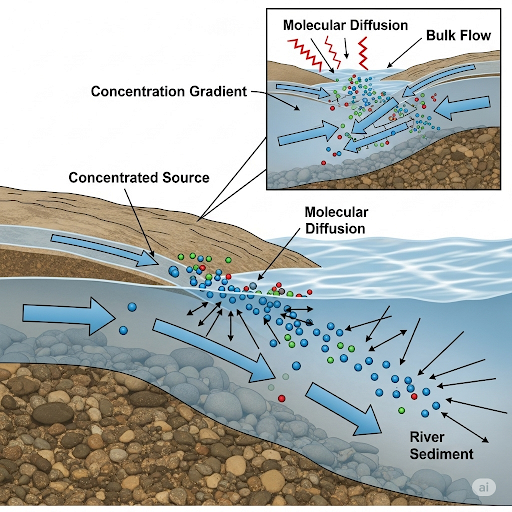
In summary, understanding the interplay between molecular diffusion and bulk flow is essential for effective environmental monitoring and pollutant management.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Molecular Diffusion and Bulk Flow
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Today, we will continue with the discussion of the fundamentals of transport. So, last class, we had discussed the issue of molecular diffusion, the flux of any species A generally is the combination of velocity and the concentration \( j = j_{bulk} + j_{diff} \). So if you are looking at the concentration, so we will make it a generic thing, so we call it as \( j_{A} \), so, i is 1, 2 for air or water okay plus j_{A}.
\( \Phi = j_{bulk} + j_{diff} \)
The term \( j_{diff} \) is the molecular diffusion term. So, in any physical scenario from the combination of two, you can decide whether the bulk flow \( j_{bulk} \) is almost zero, negligible, which means there is no bulk flow, there is no velocity.
Detailed Explanation
In this chunk, we introduce the basics of mass transfer, which includes two primary mechanisms: molecular diffusion and bulk flow. Molecular diffusion occurs when particles move from an area of high concentration to an area of low concentration, while bulk flow refers to the movement of substances due to fluid velocity. The overall flux, or flow of species A, can be understood as the sum of the movements caused by these two mechanisms. When there is little or no bulk flow, molecular diffusion dominates, whereas when bulk flow is significant, it may overshadow the effects of diffusion.
Examples & Analogies
Imagine the aroma of freshly baked cookies in your kitchen. Initially, the cookie smell is concentrated around the oven. As time passes, the scent spreads throughout your home. This spread is due to molecular diffusion where the scent moves from a high concentration (near the cookies) to lower concentrations (the rest of your kitchen). However, if you open a window and a breeze comes in (bulk flow), the scent will travel even faster through your home due to the moving air.
Scenarios of Mass Transfer
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, there are certain scenarios in the environment where the bulk flow is negligible or advection is negligible and there are scenarios where the bulk flow, \( j_{bulk} \) is much greater than molecular diffusion, \( j_{diff} \) and this implies that the \( j = j_{bulk} \). So, this diffusion is very small compared to the bulk flow, which is usually true if bulk flow exists.
Scenarios:
a) \( j_{bulk} \approx 0 \Rightarrow j = j_{diff} \), bulk flow or advection is negligible.
b) \( j_{bulk} \gg j_{diff} \Rightarrow j = j_{bulk} \), molecular diffusion is negligible.
Detailed Explanation
This chunk discusses two critical scenarios that affect mass transfer. In the first scenario, bulk flow is negligible (like a calm lake on a still day), meaning that molecular diffusion is responsible for the movement of substances. In the second scenario, bulk flow is significant (like a flowing river), which means that diffusion plays a minimal role in substance movement. Understanding these scenarios helps predict how pollutants or chemicals will move in different environmental contexts, such as lakes, rivers, and other water bodies.
Examples & Analogies
Think of a deep lake in the summer where the surface water heats up and releases evaporated moisture into the air. Here, if there's little to no wind (negligible bulk flow), substances on the lake's surface will disperse only through diffusion. Conversely, during a storm, with strong winds creating waves, the water rapidly mixes, which represents high bulk flow. The storm's movement will transport substances throughout the lake much faster than if only diffusion were at play.
Mass Transfer in Different Environmental Systems
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In systems like rivers where there is flow, the flow is significant, this is most likely true. The second case is most likely true. Is there any system that the first one is true? Any environmental system where the first one, where the advection or bulk flow is very small, the diffusion is what primarily drives mass transfer? Can you think of any environmental system? We have talked about a lot of cases where pollutant transport is important, chemical transfer is important...
So there is advection because of wind, wind induced circulation can happen and thermal convection can occur.
Detailed Explanation
This chunk covers how mass transfer varies in different environmental systems, such as rivers, lakes, and oceans. In rivers, the flow is usually significant, making diffusion less relevant. However, in systems like still lakes or deep oceans, diffusion can drive mass transfer when bulk flow is minimal. This helps us understand how pollutants behave in various environments, depending on their unique physical and chemical properties.
Examples & Analogies
Consider a quiet pond compared to a rushing river. In the pond, if you drop some dye, you will see the color spread out slowly, primarily due to diffusion. In the rushing river, if you drop the same dye, it will move quickly downstream due to the bulk flow of the water. This illustrates how the environment can dramatically influence mass transfer processes.
Mass Transfer Mechanisms in Lakes
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, it depends on the depth of the lake and the season. While we are at it, we can discuss it quickly. So, if there is a lake, this is a deep lake, very deep lake, and there is wind...
So, this is what is called as a stratified lake. The lake is stratified. So, you have one region of the lake which is well mixed, the other region is not well mixed.
Detailed Explanation
This chunk explains how lakes can be stratified, with different mixing patterns depending on their depth, wind influence, and temperature gradients. In deeper lakes, the wind primarily affects the surface layer, leading to good mixing there, while the deeper layers may remain stagnant or poorly mixed. Understanding stratification helps us predict how substances will distribute in lakes, influencing ecological health and pollutant management.
Examples & Analogies
Imagine a large smoothie where the top layer is well-blended strawberries and bananas, while the bottom remains unmixed, sticking to the bottom of the blender. This resembles how stratified lakes behave – the top area mixes sharply with wind while the bottom stays unmoved until stirred, with nutrients or substances traveling slowly through diffusion.
Key Concepts
-
Molecular Diffusion: The process by which substances move due to concentration differences.
-
Advection: Movement of substances carried by bulk fluid flow.
-
Importance of Temperature: Temperature gradients can influence mass transfer efficiency in environments.
Examples & Applications
In a deep lake during winter, cooling surface water initiates convection, and diffusion predominates at lower depths.
Groundwater flows slowly through fine soil types, making diffusion the primary transport mechanism for pollutants.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When waters flow and temps do glow, Mass transfer makes pollutants go.
Stories
In a deep lake during winter, a cold spell cools the surface water. The warmth below causes a stable layer, allowing molecules to diffuse slowly through the depths, teaching that temperature controls movement.
Memory Tools
Remember: ADVection = Air & Water movement that carries mass away quickly.
Acronyms
D.A.W.N (Diffusion Always Wins when Advection's Natural)
Reflects the conditions where diffusion can predominate.
Flash Cards
Glossary
- Mass Transfer
The movement of species from one location to another, usually driven by concentration gradients.
- Molecular Diffusion
The process by which molecules move from areas of high concentration to low concentration without bulk flow.
- Advection
The transport of substances by the bulk movement of the fluid in which they are contained.
Reference links
Supplementary resources to enhance your learning experience.
