Transport Mechanisms in Groundwater
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Transport Mechanisms
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are diving into how we move materials within groundwater systems. Can anyone tell me the two primary mechanisms of transport?

Is it molecular diffusion and advection?

Exactly! Molecular diffusion is when particles move from high to low concentration. Can anyone give me an example where diffusion is the main process?

Maybe in a deep lake where there isn’t much flow?

Great observation! In deep lakes, the chemical might primarily diffuse downwards without significant advection.

What about when there’s wind?

Good point! Wind can induce bulk flow, making advection more significant. Remember: **'DAD' for Diffusion And Drift**—it’s a neat way to remember the conditions of both processes!

So, if advection is strong, diffusion becomes less relevant?

Correct! When advection is greater, we primarily consider it in transport equations.

Let’s recap: Diffusion happens when there’s a concentration gradient without flow, while advection moves materials with the flow of water!
Effect of Soil Type on Groundwater Transport
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s consider how different soil types affect groundwater flow. Why might water flow differently in sandy soil compared to clay?

I think sandy soil is more porous, allowing for faster flow.

Exactly! Sandy soils have larger particles which allow larger spaces for water to flow quickly, while clay retains water due to its smaller particle size.

So if we have a contaminant introduced into sandy soil, it’ll move faster?

Yes! But remember, the flow velocity is still relatively slow. Let’s use the acronym **'FAST'** to remember: For Advection Speed in Transport—it's fast in sandy soils but always consider the slow overall flow effect.

What happens when we look at different environmental systems like rivers or lakes?

That's a great segue! In rivers, we often see strong advection, while in lakes, it depends on depth and external factors. Key point: **'Rivers Run Fast' and **'Lakes Linger Low.'**

To summarize, soil type hugely influences groundwater transport. Sandy is fast while clay influences retention.
Contaminant Transport Dynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving deeper, let's analyze how contaminants move through groundwater. What factors do we consider for effective transport?

It seems like diffusion and advection are key, but can you explain how they interact?

Definitely! Contaminants can move by both processes simultaneously, particularly in saturated zones. Think of diffusion as the slow, creeping cat, while advection is the speedy hare.

And in unsaturated zones, would it be mostly diffusion?

Right! In unsaturated zones, advection is limited. Contaminants primarily move through diffusion due to moisture retention. I recommend remembering: **'DIFFUSE' = DIFFusion in Upper Soil Environments.**

What about sediments? How would contaminants behave there?

Great question! In sediments, diffusion is usually the main process again, as water movement is minimal there unless gases form through biological reactions which can create some advection.

In conclusion, remember, contaminants navigate mainly through diffusion in unsaturated zones and sediments, contrasting with stronger advection in saturated environments.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Groundwater transport mechanisms are primarily governed by two processes: molecular diffusion and bulk flow, also known as advection. These processes dictate how contaminants move through groundwater, particularly in different geological contexts such as lakes, rivers, and saturated versus unsaturated soils.
Detailed
Transport Mechanisms in Groundwater
Groundwater transport involves complex interactions between contaminants and the water in subsurface environments. Two primary mechanisms govern this transport:
- Molecular Diffusion: This process is driven by concentration gradients, where molecules move from areas of high concentration to low concentration, even when there is no bulk flow.
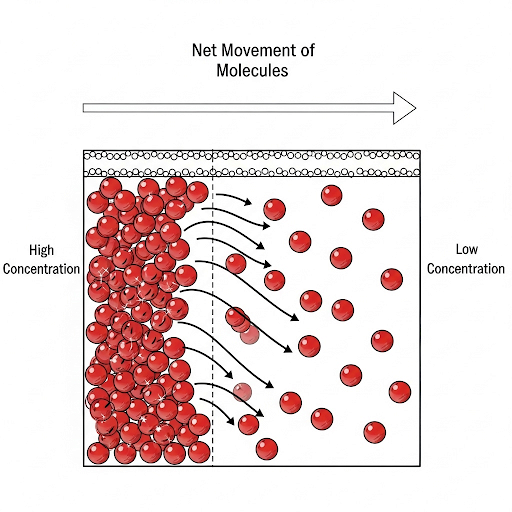
- Advection or Bulk Flow: This refers to the movement of water due to pressure gradients, often caused by gravity or external forces like wind. In ecosystems such as lakes or rivers with significant flow, advection typically dominates.
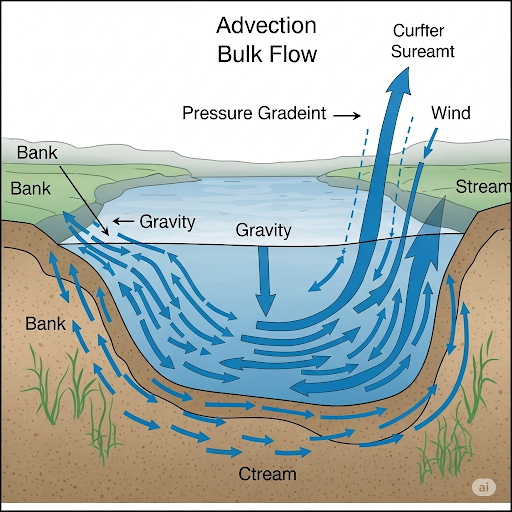
In many groundwater scenarios, either advection may be negligible (allowing diffusion to play a dominant role) or advection may vastly outweigh diffusion, particularly in well-mixed systems like rivers. Moreover, the nature of the soil (sandy vs. clay) significantly influences the flow velocity and the behavior of contaminants.
Environmental contexts, such as stratified lakes or saturated zones, illustrate these transport processes. For instance, pollutants introduced at the surface of a deep lake will predominantly diffuse downwards due to concentration gradients unless significant wind-induced circulation alters the flow. These dynamics become critical in understanding pollutant transport within integrated soil-water systems.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Groundwater Flow
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
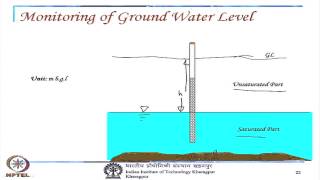
Groundwater essentially means it is soil, we are talking about soil systems. So, soil systems, there is a water table and there is an aquifer, these are porous media. If there is a chemical that is sitting here, this chemical then moves in this direction, where there is a velocity in the direction of the gradient of the groundwater.
Detailed Explanation
Groundwater refers to the water held underground in soil or rock. In a typical soil system, we have layers: unsaturated (air-filled) soil above and saturated soil (filled with water) below, along with a water table that separates the two. When a chemical is present in this groundwater, it moves due to the velocity created by gradients in pressure or concentration; meaning, the chemical moves from areas of high concentration to low concentration, i.e., following the flow pattern of the groundwater.
Examples & Analogies
Imagine a sponge filled with water. If you squeeze one end of the sponge, the water closer to that end will move towards the other end like how chemicals move in groundwater when pressure is applied.
Velocity of Groundwater Flow
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This velocity varies from place to place depending on whether there is clay soil, sandy soil, and all that, geology of that region matters a lot. The velocity of groundwater changes, but it is very small.
Detailed Explanation
The speed at which groundwater moves can be influenced by the type of soil it is in. Sandy soils allow water to flow more quickly due to larger pore spaces, while clay soils are packed tighter, restricting flow and slowing it down. As a result, groundwater flow is typically very slow compared to rivers, for example.
Examples & Analogies
Think of it like a crowded highway (clay soil) where cars move slowly due to congestion, versus an open freeway (sandy soil) where vehicles can speed along without obstacles.
Importance of Both Diffusion and Flow
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In groundwater flow, both advection (bulk flow) and molecular diffusion are important, so you cannot neglect flow or diffusion. Both have to be considered at the same time.
Detailed Explanation
In groundwater transport, it is essential to consider both how quickly the water (and any dissolved chemicals) moves due to flow (advection) and how molecules spread out naturally from areas of high concentration to areas of lower concentration (diffusion). This means that while groundwater might be moving slowly, chemicals can still be transported through molecular diffusion.
Examples & Analogies
Think of a tea bag in a cup of hot water. The water (bulk flow) moves the tea flavors quickly throughout the cup, but in areas where the water is still (say, the very center), the flavors diffuse into the water slowly, making sure the whole cup of tea gets the flavor over time.
Scenarios of Dominant Transport Mechanisms
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Depending on the environmental conditions, there can be instances where advection is negligible and diffusion becomes the primary mode of transport. For example, in the unsaturated zone, diffusion is often the main mechanism since there may not be enough water for significant flow.
Detailed Explanation
In environments where the soil does not have enough moisture, like the unsaturated zone, movement of contaminants or chemicals relies primarily on diffusion. The movement is very slow and is driven by concentration gradients, where substances migrate from areas of high concentration to areas of low concentration because water flows are insufficient to transport them.
Examples & Analogies
Picture sugar scattered over dry soil. Even without water to move it, over time, the sugar will spread out evenly across the surface due to diffusion, albeit very slowly.
Chemical Movement in Sediments
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In sediment, chemicals can move down through diffusion as there is often no flow present. This can lead to chemical transport being detected much later, often decades after contamination.
Detailed Explanation
In sediments at the bottom of lakes and rivers, water may not flow much, and this limits how quickly contaminants can move. Instead, they diffuse slowly, and thus, any change or detection of these chemicals can take a very long time, as they spread out over years and decades.
Examples & Analogies
Imagine putting a drop of food coloring in a glass of still water. If you don't stir, you'll see that the coloring diffuses slowly throughout the water, but it takes time. If you wait, eventually the water will be evenly colored, but it won’t happen immediately.
Key Concepts
-
Molecular Diffusion: The movement from high to low concentration.
-
Advection: Bulk movement of water carrying substances.
-
Saturated vs. Unsaturated Zones: Impacting transport mechanisms.
-
Soil Type Influence: Different soils affect flow velocities.
-
Contaminant Dynamics: Interplay between diffusion and advection.
Examples & Applications
In a deep lake on a still day, a pollutant introduced would primarily diffuse due to negligible bulk flow.
In sandy soil, contaminants may move rapidly through advection, while in clay soil, movement would be slower with higher retention of contaminants.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the soil, the waters flow, diffusion's slow but advection's a show!
Stories
Imagine a river and lake talking. The river boasts about how fast it carries leaves, while the lake explains how slowly perfume spreads at its depths, teaching us how different systems move materials.
Memory Tools
Use 'MDA' for Molecular diffusion Dominates in Advemming environments.
Acronyms
Remember **'FAST'** for water in sandy soil—**F**ast movement; **A**dvecting is significant; **S**low in clay; **T**ransport varies.
Flash Cards
Glossary
- Molecular Diffusion
The process where molecules move from areas of high concentration to low concentration.
- Advection
The transport of a substance by the bulk motion of a fluid.
- Bulk Flow
The movement of mass in the direction of flowing water, similar to advection.
- Saturated Zone
The area in soil or rock where all the pores are filled with water.
- Unsaturated Zone
The area above the groundwater table where soil pores contain both air and water.
- Contaminant Transport
The movement of pollutants through environmental media, including groundwater.
- Hydraulic Gradient
The change in hydraulic head per unit distance, influencing groundwater flow direction.
- Porosity
The percentage of void spaces in a material, indicating its ability to hold water.
- Conductivity
The ability of soil to transmit water, influencing flow rate.
Reference links
Supplementary resources to enhance your learning experience.
