Chemistry of Ozone Depletion
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ozone and its Importance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re discussing ozone and why it’s crucial for life on Earth. Can anyone tell me what ozone actually does?

Ozone protects us from harmful UV radiation from the sun.

Exactly! Ozone acts like a shield, filtering out damaging UV-B rays. Can someone explain where ozone is primarily found?

Most ozone is found in the stratosphere, right?

Correct! The stratosphere is where the ozone layer exists, typically between 10 and 50 km above the Earth. Remember the acronym 'UV Be Gone' to recall the protection ozone provides against UV rays!

Does the ozone layer ever get damaged?

Yes, it does! Human-made chemicals are a major cause. Let’s explore how this happens.
Ozone Depletion Process
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Ozone depletion primarily occurs due to substances like CFCs. Can anyone say how these chemicals reach the stratosphere?

They are released from products like fridges and aerosols.

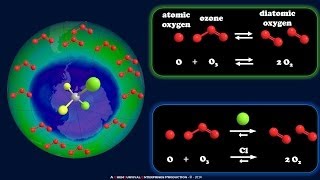
Exactly! They’re stable and don’t break down easily, allowing them to reach the stratosphere. What happens once they’re up there?

UV light breaks them apart, releasing chlorine atoms.

Great! Each chlorine atom can destroy thousands of ozone molecules. We can remember this impact using the phrase 'One to Many', representing one chlorine’s effect on many ozone molecules. What’s next?

The ozone molecules get split into oxygen molecules and chlorine monoxide.

Yes! And it can continue the cycle, further depleting ozone. Understanding this cycle helps us grasp the seriousness of the problem.
Effects of Ozone Layer Depletion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about the effects of ozone depletion. What impact do you think increased UV radiation could have on human health?

It could lead to more skin cancers and eye problems.

Absolutely! Skin cancer is a major concern, alongside cataracts. How about the environment? Any thoughts?

It could harm plants and disrupt ecosystems.

Exactly! Increased UV can alter species composition in forests and affect aquatic life like phytoplankton, which are foundational to food webs. To remember, think of 'Sun’s Rage – a dark age for nature' as a reminder of the negative impacts.

What about air quality?

Good question! Ozone depletion affects air quality by increasing photochemical reactions, which can produce pollutants harmful to both health and the environment.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The chemistry behind ozone depletion involves the breakdown of ozone (O3) molecules by chlorine and bromine compounds found in chlorofluorocarbons (CFCs) and other ozone-depleting substances. This depletion results in increased ultraviolet radiation reaching the Earth, causing harm to human health, ecosystems, and biodiversity.
Detailed
Detailed Summary of Chemistry of Ozone Depletion
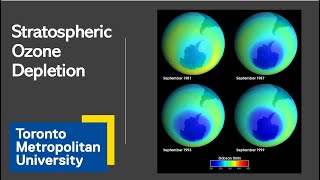
Ozone (O3) plays a crucial role in filtering harmful UV-B radiation from the sun. However, the balance of ozone production and destruction in the stratosphere has been disrupted primarily by human-made chemicals such as chlorofluorocarbons (CFCs). When CFCs are released into the atmosphere, they eventually diffuse into the stratosphere where they are broken down by ultraviolet (UV) light, releasing chlorine atoms. One chlorine atom can destroy up to 100,000 ozone molecules before being removed from the stratosphere. This results in thinning of the ozone layer, particularly marked by the seasonal formation of the 'ozone hole' over the poles.
The consequences of ozone depletion include increased radiation exposure to humans and animals, potentially leading to higher rates of skin cancer, eye cataracts, and other health issues. Moreover, ecosystems, particularly aquatic life and terrestrial plants, suffer from altered growth patterns and biodiversity loss. Overall, ozone depletion has substantial implications on health, ecosystems, and global processes.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Consequences of Ozone Depletion
Chapter 1 of 1
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ozone layer, therefore, is highly beneficial to plant and animal life on earth filtering out the dangerous part of sun’s radiation and allowing only the beneficial part to reach earth. Any disturbance or depletion of this layer would result in an increase of harmful radiation reaching the earth’s surface leading to dangerous consequences.
Detailed Explanation
The ozone layer plays a critical role in protecting life on Earth by blocking harmful ultraviolet (UV-B) radiation from the sun. This radiation is known to cause significant health issues such as skin cancer, cataracts, and diminished immune responses. It can also harm terrestrial plants and marine ecosystems. As the ozone layer gets depleted, more UV radiation can reach the Earth’s surface, resulting in adverse effects on human health, plant ecosystems, and aquatic life, as well as contributing to environmental changes.
Examples & Analogies
Think of the ozone layer as a pair of sunglasses protecting your eyes from harsh sunlight. Without these sunglasses, your eyes become vulnerable to bright light that can cause damage. Similarly, the ozone layer protects living organisms from harmful UV radiation, and if it weakens, the consequences can be dire, just like forgetting to wear sunglasses on a sunny day can lead to painful eye strain or long-term vision problems.
Key Concepts
-
Ozone Depletion: The reduction of ozone in the stratosphere due to chemical reactions with human-made substances.
-
Chlorofluorocarbons (CFCs): Compounds that release chlorine when broken down by UV radiation, significantly contributing to ozone depletion.
-
UV Radiation: Increased levels of ultraviolet radiation can lead to serious health risks and environmental impacts.
Examples & Applications
Ozone layer depletion has led to increased rates of skin cancer in regions with high UV exposure due to the thinning ozone layer.
Phytoplankton, which are vital to aquatic food webs, experience altered distributions and population declines due to increased UV radiation.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ozone layer up high, keep the UVs from my eye.
Stories
Once there was a protective shield called ozone, but mischievous CFCs broke it down, letting harmful rays through and causing troubles in the world like skin burns and sick sea creatures.
Memory Tools
CFCs Lead to Ozone Reduction: 'CLOSER' - Chlorine from CFCs Leads to Ozone's Reduction.
Acronyms
Remember the acronym 'CFC' to connect it with 'Chlorofluorocarbons' and 'Ozone Depletion'.
Flash Cards
Glossary
- Ozone (O3)
A molecule composed of three oxygen atoms, primarily found in the stratosphere, that absorbs harmful UV radiation.
- Chlorofluorocarbons (CFCs)
Human-made compounds containing chlorine, fluorine, and carbon that contribute significantly to ozone depletion.
- UV Radiation
Ultraviolet radiation from the sun that can cause harm to living organisms, particularly at higher levels.
- Stratosphere
The layer of the Earth's atmosphere located above the troposphere, where the ozone layer is situated.
- Ozone Hole
A seasonal depletion of ozone observed primarily over Antarctica due to human-made chemicals.
Reference links
Supplementary resources to enhance your learning experience.
